Cristallizzazione dell'acido salicilico mediante modificazione chimica
Panoramica
Fonte: Kerry M. Dooley e Michael G. Benton, Dipartimento di Ingegneria Chimica, Louisiana State University, Baton Rouge, LA
L'elaborazione di sostanze biochimiche comporta operazioni unitarie come la cristallizzazione, l'ultracentrifugazione, la filtrazione a membrana e la cromatografia preparativa, che hanno tutte in comune la necessità di separare le molecole grandi da piccole o solide da liquide. Di questi, la cristallizzazione è la più importante dal punto di vista del tonnellaggio. Per questo motivo, è comunemente impiegato nelle industrie farmaceutiche, chimiche e di trasformazione alimentare. Importanti esempi biochimici includono separazioni chirali,1 purificazione di antibiotici,2 separazione di amminoacidi dai precursori,3 e molti altri prodotti farmaceutici,4-5 additivi alimentari,6-7 e purificazioni agrochimiche. 8 Il controllo della morfologia dei cristalli e della distribuzione dimensionale è fondamentale per l'economia di processo, in quanto questi fattori influenzano i costi delle operazioni di lavorazione a valle come l'essiccazione, la filtrazione e il trasporto dei solidi. Per ulteriori informazioni sulla cristallizzazione, consultare un libro di testo specializzato o un libro di testo Unit Operations. 9 anni
L'unità cristallizzante (Figura 1) consente di studiare: (a) gli effetti di parametri chiave, quali la sovrasaturazione e le velocità di raffreddamento/riscaldamento, sul contenuto di solidi, sulla morfologia e sulla distribuzione dimensionale dei cristalli; b) e il controllo in linea dei processi di cristallizzazione. La sovrasaturazione può essere controllata alterando condizioni come la velocità di agitazione e la temperatura. Le diverse classificazioni della cristallizzazione includono raffreddamento, evaporazione, oscillazione del pH e modifica chimica. In questo esperimento, un microscopio offline misurerà da cristalli di dimensioni variabili da 10-1000 μm, una gamma di dimensioni tipiche per i biologici.

Figura 1: Schema P&ID (a sinistra) e immagine (a destra) di Crystallizer. Fare clic qui per visualizzare una versione più grande di questa figura.
Questo esperimento dimostrerà una "modificazione chimica", o cristallizzazione "pH-swing", per generare cristalli di acido salicilico (SAL) (precursore dell'aspirina) dalla reazione rapida di soluzioni acquose di salicilato di sodio basico (NaSAL), che sono basici, e acido solforico (H2SO4) ovunque da 40 - 80 ° C.11
Na+SAL + 0,5 H2SO4 SAL (ppt) + Na+ + 0,5 SO42-
Il sottoprodotto solfato di sodio rimane solubile. L'apparecchio è costituito da due serbatoi di alimentazione, tre pompe a velocità variabile (peristaltiche), il cristallizzatore (serbatoio agitato per approssimare temperatura e concentrazione uniformi, ~ 5 L), un bagno circolante per il controllo della temperatura, un regolatore di potenza, un serbatoio del prodotto e un serbatoio di trucco per la rigenerazione dell'alimentazione con soluzione NaOH (se lo si desidera). I campioni saranno analizzati da uno spettrometro UV-Vis per lo ione salicilato solubile residuo e il prodotto cristallino di acido salicilico sarà essiccato e pesato. Una sonda di pH può essere utilizzata per determinare lo stato stazionario quando le condizioni di reazione sono alterate.
Procedura
Le soluzioni organiche (salicilato di sodio, NaSAL) e acide (acido solforico, 0,25 M = 0,50 N) saranno alimentate al cristallizzatore. Assicurarsi di indossare guanti in lattice quando si maneggia NaSAL, acido salicilico o le loro soluzioni e acido solforico 0,25 M.
L'intero sistema è controllato da un PC utilizzando un controller distribuito commerciale con un'interfaccia simile a quella della Figura 1. Tutte le elettrovalvole on-off o a 3 vie e i set point del c
Risultati
La Figura 2 presenta dati rappresentativi che suggeriscono deviazioni modeste dalla distribuzione dimensionale cristallina dell'ideale MSMPR anche a velocità relativamente elevate e basse concentrazioni di alimentazione.

Figura 2. Distribuzione cristallina per alimentazione NaSAL 0,16 M, 540 giri/min, 60 °C
I cristalli ch..
Applicazione e Riepilogo
Questo esperimento ha dimostrato come effettuare misurazioni grezze di concentrazione, flusso e temperatura e utilizzare la teoria MSMPR per stimare i parametri chiave necessari per progettare un sistema di cristallizzatori grande e complesso. È stato esplorato il ruolo critico che il tempo di permanenza gioca nell'ottenere alte rese di cristallo e nel controllare la dimensione media dei cristalli. Spesso c'è un tempo di residenza ottimale perché raramente sono desiderabili cristalli molto grandi. Lo stesso vale per l...
Riferimenti
- C. Wibowo, L. O’Young and K.M. Ng, Chem. Eng. Prog., Jan. 2004, pp. 30-39.
- W.J. Genck, Chem. Eng. Prog., Oct. 2004, pp. 26-32.
- S. Takamatsu and D.D.Y. Ryu, Biotechnol. Bioeng., 32, 184-191 (1988).
- F. Wang and K.A. Berglund, Ind. Eng. Chem. Res., 39, 2101-2104 (2000).
- Y. Kim, S. Haam, Y.G. Shul, W.-S. Kim, J.K. Jung, H.-C. Eun and K.-K. Koo, Ind. Eng. Chem. Res., 42, 883-889 (2003).
- K. Hussain, G. Thorsen and D. Malthe-Sorenssen, Chem. Eng. Sci., 56, 2295-2304 (2001).
- H. Gron, A. Borissova and K.J. Roberts, Ind. Eng. Chem. Res., 42, 198-206 (2003).
- F. Lewiner, G. Fevotte, J.P. Klein and F. Puel, Ind. Eng. Chem. Res., 41, 1321-1328 (2002).
- For example: W.L. McCabe, J.C. Smith, and P. Harriott, “Unit Operations of Chemical Engineering”, 7th Ed., McGraw-Hill, New York, 2005, Ch. 27, or C.J. Geankoplis, “Transport Processes and Unit Operations”, 3rd Ed., 1993, Ch. 12.
- P. Barrett, Chem. Eng. Prog., Aug. 2003, pp. 26-32.
- R. Franck, R. David, J. Villermaux and J.P. Klein, Chem. Eng. Sci., 43, 69-77 (1988).
- J. Garside, Chem. Eng. Sci., 40, 3-26 (1985).
- H. Zhao, J.-X. Wang, Qi-An Wang, J.-F. Chen and J. Yun, Ind. Eng. Chem. Res. 46, 8229-8235 (2007).
- J.S. Kwon, M. Nayhouse, G. Orkoulas and P.D. Christofides, Ind. Eng. Chem. Res., 53, 15538-15548 (2014).
Vai a...
Video da questa raccolta:

Now Playing
Cristallizzazione dell'acido salicilico mediante modificazione chimica
Chemical Engineering
24.3K Visualizzazioni

Verifica dell'efficienza del trasferimento di calore di uno scambiatore di calore a tubi alettati
Chemical Engineering
17.9K Visualizzazioni

Utilizzo di un essiccatore a vassoio per studiare il trasferimento di calore convettivo e conduttivo
Chemical Engineering
44.0K Visualizzazioni

Viscosità delle soluzioni di glicole propilenico
Chemical Engineering
33.0K Visualizzazioni

Porosimetria della polvere di silicato di alluminio
Chemical Engineering
9.6K Visualizzazioni

Dimostrazione del modello Power Law per estrusione
Chemical Engineering
10.2K Visualizzazioni

Assorbitore di gas
Chemical Engineering
36.8K Visualizzazioni

Equilibrio vapore-liquido
Chemical Engineering
89.3K Visualizzazioni

L'effetto del rapporto di riflusso sull'efficienza della distillazione dei vassoi
Chemical Engineering
77.8K Visualizzazioni

Efficienza di estrazione liquido-liquido
Chemical Engineering
48.5K Visualizzazioni

Reattore in fase liquida: inversione del saccarosio
Chemical Engineering
9.7K Visualizzazioni

Flusso monofase e bifase in un reattore a letto impaccato
Chemical Engineering
19.0K Visualizzazioni

Cinetica di polimerizzazione per addizione al polidimetilsilossano
Chemical Engineering
16.1K Visualizzazioni

Reattore catalitico: Idrogenazione dell'etilene
Chemical Engineering
30.5K Visualizzazioni

Valutazione del trasferimento di calore di uno Spin-and-Chill
Chemical Engineering
7.4K Visualizzazioni
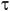 (tempo di residenza) variando le portate volumetriche, ma mantenendo l'equivalenza stechiometrica.
(tempo di residenza) variando le portate volumetriche, ma mantenendo l'equivalenza stechiometrica.