Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Isolation and Derivation of Mouse Embryonic Germinal Cells
W tym Artykule
Podsumowanie
The ability of embryonic germinal cells to differentiate into primordial germinal cells during early development stages is a perfect model to address our hypothesis about cancer and infertility. This protocol shows how to isolate primordial germinal cells from developing gonads in 10.5-11.5 days post coitum mouse embryos.
Streszczenie
Protokół
Part 1: Pregnant Mouse Laparotomy
- Using cervical dislocation, euthanize a pregnant C57BL6J female mouse at 10.5-11.5 dpc.
- Clean the abdomen with antimicrobial soap, and then, shave it.
- After shaving, wash the abdomen with a saline solution.
- Then, dry the abdomen with a sterile gauze.
- Cover the mouse with a new sterile field.
- Make a ventral incision using forceps and dissection scissors.
- Identify and remove the entire uterus from the abdominal cavity. Mouse embryos will be visible inside the uterus.
- Transfer the uterus into a petri dish filled with D-PBS, and keep it on ice.
- Using a sterile scalpel and forceps, remove the placenta and extra embryonic tissues of each embryo.
- Transfer embryos into a fresh petri dish filled with D-PBS.
- Measure the length of the mouse embryos. We found that the sizes differed according to the embryo’s age. For example 8.5 dpc measured at an average of 6mm, 10.5 dpc measured at an average of 11mm, and 12.5 dpc measured at an average of 16 mm.
- Then, remove their tails for DNA extraction.
Part 2: Gonadal Ridge Dissection
Under stereomicroscope and Light source Schott Fostec
- Place a filter paper in a Petri dish. Then, place the mouse embryo on top of the filter paper to dry the embryo and immobilize for dissection.
- Using a sterile scalpel, make a transverse cut of the mouse embryo above the origin of the umbilical cord.
- Under a stereomicroscope, using sterile fine forceps and a sterile fine teasing needle, remove the intestines and liver so that the urinary system is visible.
The kidneys are located laterally in the abdominal cavity, and the bladder is located medial and caudal in comparison to the kidneys. The embryo can be identified as male because the gonadal ridges are located on either side of the bladder. In the female, the gonadal ridges are firmly attached to the caudal lateral end of the kidneys. - Peel the gonadal ridge out by sliding a needle behind it. The gonadal ridge is removed by cutting away the tissues that support it.
- Transfer the gonadal ridges to a new petri dish filled with fresh D-PBS.
Microscopically, the male gonadal ridge should be stripped, large and oval in shape. In contrast, the female gonadal ridge should be spotted, have elongated shape and be smaller in comparison to the male gonadal ridge.
Part 3: Gonadal Ridge Digestion
Under stereomicroscope:
- Mesonephros Dissection: Separate the gonad from the mesonephros and ridge using a fine and sharp needle.
- It is important to remove the mesonephros from the gonadal ridge because it is a somatic tissue that will overgrow in the PGC derivation process.
- Gonadal Ridge Digestion: Collect clean gonadal ridges in a 0.5ul drop of fresh D-PBS. Add 20 ml of Collagenase/Dispase Solution (Final concentration at 1mg/ml).
- Embryos should be processed individually. Each embryo should have its own separate and labeled petri dish.
- Gonadal Ridge Mincing: Cut the gonadal ridge into small pieces using a sterile needle and sterile fine forceps No. 4.
- Incubation: Petri dishes are transferred to an incubator at 37°C for 15 minutes.
- The incubation time could vary between tissues.
- Pipetting: After 15 minutes, the tissue can be dissociated by pipetting. Using pulled glass capillary pipettes with diameters between 40 -100 mm Break up the pieces by pipetting up and down. This will form small clumps. Transfer the clumps to 0.5ml of a pre warmed D-PBS drop for a final wash.
- After washing, transfer the clumps to eppendorf tubes.
- Do not over expose the tissue to collagenase.
- Do not make a single cell suspension. This is important for colony formation. If it is a single cell suspension, PGCs tend to differentiate and migrate.
- This process should not be longer than 20 minutes, or your cells will lose viability.
- Centrifuge at 2000 rpm for 5 minutes at room temperature.
- After centrifuging, remove the supernatant, and resuspend the pellet in 0.5 ml of supplemented knock out media (Pre-warmed at 37°C).
Part 4: Primordial Germinal Cells Culture
"You should perform the next steps quickly to preserve the viability of PGCs for isolation and derivation.
- 24 hours before this process you should prepare a mitotically inactivated MEF-CF1 feeder layer following the protocol from Zhang J. et al 2008.
- Prepare 20 dishes for one pregnant female mouse (6-8 embryos).
- MEFs lose the ability to be a feeder layer (promote growth and prevent differentiation) after 5-6 passages.
- Do not use MEFs older than 48 hours.
- Immediately before plating PGCs, the MEF medium must be slowly removed from the MEF feeder layer.
- Then, add 0.5 ml/well of pre-warmed supplemented knockout medium onto the MEF feeder layer.
- MEF media contains fetal bovine serum that induces differentiation of PGCs.
- Knockout medium must be supplemented with specie specific growth factors immediately before use to ensure the activity of the grow factor.
- Using symmetrical distribution, plate PGCs over the MEF feeder layer.
- If these pieces are too close, they tend to aggregate and make dense colonies that attach poorly or begin differentiating.
- Culture dishes must be labeled with Embryonic Germinal cell line name, passage number, and date.
- Carefully, transfer the culture dishes into an incubator at 37°C and 5% CO2.
- Monitor the cells every 24 hours.
- After 48 hours, remove 250 ml of media from the very top of the culture to avoid disturbing the colony's attachment. Then, add 250 ml of fresh supplemented knock-out media (pre-warmed at 37°C).
- After step 7, wait another 48 hours, and then, completely remove all media and replace it with fresh supplemented knock-out media (pre-warmed at 37°C). Do this every 2 days for 8-10 days until PGC colonies form.
Part 5: Embryonic Germinal Cells
- After 10 days of PGC isolation, embryonic germinal cell colonies are formed.
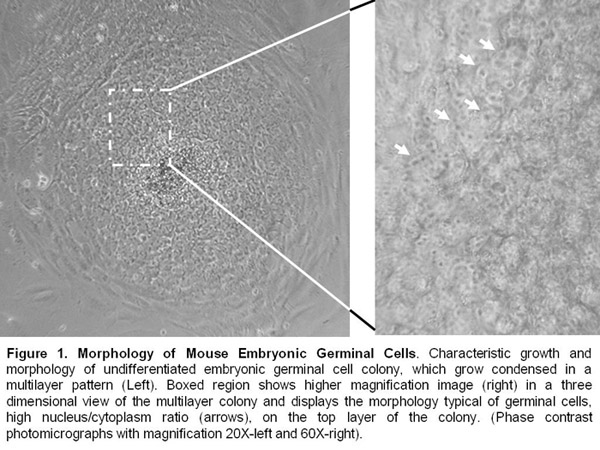
- Embryonic germinal cell lines are kept alive by manual passages every 8-10 days. They continue to present an undifferentiated morphology and express pluripotency markers such as alkalyne phosphatase, Oct-4, SSEA-1, and SOX-2
Notes
- Sterile/aseptic conditions are essential in the culture room.
- For derivation and culture, PGCs are processed in a Purifier Class II Bio safety Cabinet.
- Incubations are in a humidified 37°C and 5% CO2 incubator.
- All media and reagents are filtered prior to use in a 0.2 um filter, stored at 4°C, and pre-warmed at 37°C before use.
- During the dissection steps, all embryos and tissues must be kept on ice.
- All reagent flasks are decontaminated with ethanol before placing in a cabinet.
- Use of gloves, lab coat, and nurse caps are mandatory.
Conclusion
We have provided a video that shows you how to isolate, derive, and culture embryonic germinal cells from gonadal ridges of 10.5-11.5 dpc mouse embryos. The reproducible isolation and long term culture of Embryonic germinal cell lines provides a critical foundation for study of early embryonic development, genetic and epigenetic germinal patterns, gonad formation, environmental effects of surrounding organs during the developmental process of gametogenesis in the male and female embryos, and determination of pathways that leads to cancer and infertility.
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
This research was conducted after review and Institutional Animal Care and Use Committee (IACUC) approval of our mouse protocol #08030 (The Role of Reactive Oxygen Species -ROS in germ line development and tumor formation: Holding and breeding protocol) at Mississippi State University.
Podziękowania
The authors would like to acknowledge the invaluable help of: Dr Neal First and Kourtney Wilkinson for manuscript editing; Dr Lucy Senter, Dr. Brigit Willeford and Mike Basett for assistance with training and animal care at the MSU ALAC accredited mouse facility; Dr Dwayne Wise for his assistance with microscopy and image capture; Cesar Monroy, Hannah Swoope, and Bobbie Huddleston for their assistance with video production at MSU. This research was funded by Office of Institutional Research and the Department of Biological Sciences at Mississippi State University.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| 10.5 - 11.5 dpc pregnant C57BL6J female mouse crossed with the same background male mouse. | |||
| Mouse Fibroblast MEF-CF1 ATCC | SCRC-1040 | ||
| Dulbecco’s Phosphate Buffered Saline (D-PBS) | Invitrogen | 14190/086 | |
| Collagenase /Dispase Solution | Roche Group | 269638 | |
| Gelatin | Sigma-Aldrich | G1890 | |
| Mitomycin C | Sigma-Aldrich | M4287 | |
| Trypsin EDTA | Invitrogen | 25200-072 | |
| MEF Medium: Dulbecco Medium Eagle Modified (D-MEM) GLUTAMAX High glucose | Invitrogen | 10566024 | |
| 10% Fetal Bovine Serum | Invitrogen | 16000/044 | |
| 1% of antibiotic-antimycotic | Invitrogen | 15420/096 | |
| Knockout Media: 80% knockout D-MEM | Invitrogen | 10829/018 | |
| 20% Knockout serum Invitrogen Cat. No. 1028/028, 200mM L-glutamine Invitrogen | 25130/081 | with β-mercapt–thanol Sigma Catalogue Number: M7522 | |
| 1X non essential amino acid solution | Invitrogen | 11140/050 | |
| 1% antibiotic-antimycotic | Invitrogen | 15420/096 | supplemented with grow factor as 2500 U of Leukemia inhibitory factor (mLIF-ESGRO) Millipore Catalogue Number: ESG1106 |
| 40 ng/ml of Recombinant Murine Stem Cell Factor (SCF) ReproTech | 250-03 | ||
| 20 ng/ml of Basic Fibroblast Growth factor (bFGF) | Invitrogen | 13256-029 | |
| Corning center well culture dish 60mm | Fisher Scientific | 07-200-79 | 1.5 ml eppendorf tube, and 15 ml falcon tubes |
| Forceps No. 14 and 15, scalpel 35 mm, dissection scissors, surgical fields, antimicrobial soap, saline solution 0.9 %, gauze, ice foam container, razor blade, scalpel 35 mm, fine forceps No. 4 and 5, fine teasing needle with handle, filter paper sheets, and pulled glass pipettes. Equipment: dissecting stereomicroscope, light source Schott Fostec, inverted microscope, micropipette 10 and 200ul, centrifuge, bio safety Cabinet. | |||
Equipment: dissecting stereomicroscope, light source Schott Fostec, inverted microscope, micropipette 10 and 200ul, centrifuge, bio safety Cabinet. | |||
Equipment: dissecting stereomicroscope, light source Schott Fostec, inverted microscope, micropipette 10 and 200ul, centrifuge, bio safety Cabinet. | |||
| Equipment: dissecting stereomicroscope, light source Schott Fostec, inverted microscope, micropipette 10 and 200ul, centrifuge, bio safety Cabinet. | |||
Odniesienia
- DeFelici, M. Cell Biology: A laboratory Handbook. 1, Second Edition, 73-85 (1998).
- Donovan, P. J., De Miguel, M. P. Turning Germ Cells into Stem Cells. Current Opinion in Genetics and Development. 13, 463-471 (2003).
- Labosky, P. A., Hogan, B. L. M. Part 12: Mouse Primordial Germ Cells: isolation and in vitro culture. Molecular embryology: methods and protocols. Sharp, P. T., Mason, I. 97, Humana Press. Totowa, NJ. (1999).
- Mc Laren, A., Southee, D. Entry of Mouse Embryonic Germ Cells into Meiosis. Developmental Biology. 187, 107-113 (1997).
- Yoshimizu, T., Obinata, M., Matsui, Y. Stage-specific tissue and cell interactions play key roles in mouse germ cell specification. Development. 128, 481-490 (2001).
- Zhang, J., Hhvorostov, I., Teitell, M. From MEFs to Matrigel I: Passaging hESCs in the Presence of MEFs. J Vis Exp. , (2008).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone