Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Two Types of Assays for Detecting Frog Sperm Chemoattraction
W tym Artykule
Podsumowanie
Eggs and the extracellular coatings around eggs frequently release peptides, proteins and small molecules that communicate with sperm to guide them to the egg thereby promoting fertilization. Using frog sperm we describe and compare two classes of assays used to detect sperm chemoattraction – sperm accumulation assays and sperm tracking assays.
Streszczenie
Sperm chemoattraction in invertebrates can be sufficiently robust that one can place a pipette containing the attractive peptide into a sperm suspension and microscopically visualize sperm accumulation around the pipette1. Sperm chemoattraction in vertebrates such as frogs, rodents and humans is more difficult to detect and requires quantitative assays. Such assays are of two major types - assays that quantitate sperm movement to a source of chemoattractant, so-called sperm accumulation assays, and assays that actually track the swimming trajectories of individual sperm.
Sperm accumulation assays are relatively rapid allowing tens or hundreds of assays to be done in a single day, thereby allowing dose response curves and time courses to be carried out relatively rapidly. These types of assays have been used extensively to characterize many well established chemoattraction systems - for example, neutrophil chemotaxis to bacterial peptides and sperm chemotaxis to follicular fluid. Sperm tracking assays can be more labor intensive but offer additional data on how chemoattractancts actually alter the swimming paths that sperm take. This type of assay is needed to demonstrate the orientation of sperm movement relative to the chemoattrractant gradient axis and to visualize characteristic turns or changes in orientation that bring the sperm closer to the egg.
Here we describe methods used for each of these two types of assays. The sperm accumulation assay utilized is called a "two-chamber" assay. Amphibian sperm are placed in a tissue culture plate insert with a polycarbonate filter floor having 12 μm diameter pores. Inserts with sperm are placed into tissue culture plate wells containing buffer and a chemoatttractant carefully pipetted into the bottom well where the floor meets the wall (see Fig. 1). After incubation, the top insert containing the sperm reservoir is carefully removed, and sperm in the bottom chamber that have passed through the membrane are removed, pelleted and then counted by hemocytometer or flow cytometer.
The sperm tracking assay utilizes a Zigmond chamber originally developed for observing neutrophil chemotaxis and modified for observation of sperm by Giojalas and coworkers2,3. The chamber consists of a thick glass slide into which two vertical troughs have been machined. These are separated by a 1 mm wide observation platform. After application of a cover glass, sperm are loaded into one trough, the chemoattractant agent into the other and movement of individual sperm visualized by video microscopy. Video footage is then analyzed using software to identify two-dimensional cell movements in the x-y plane as a function of time (xyt data sets) that form the trajectory of each sperm.
Protokół
1. Materials and buffers used
- Oocyte Ringer′s Buffer (1.5 x OR2) contains 124 mM NaCl, 3.75 mM KCl, 1.5 mM CaCl2, 1.5 mM MgCl2, 1.5 mM Na2HPO4, 10 mM Hepes, pH 7.8. Fertilization Buffer (F-1) contains 41.25 mM NaCl, 1.25 mM KCl, 0.25 mM CaCl2, 0.06 mM MgCl2, 0.5 mM Na2HPO4, 2.5 mM Hepes, pH 7.8.
- Xenopus laevis egg water is prepared according Sugiyama et al.4. Briefly described, freshly spawned jellied frog eggs are swirled in a small volume of F-1 buffer for 30 minutes and the conditioned medium removed by micropipette. This medium, termed "egg water", can also be used to prepare purified allurin, the primary chemoattractant in this jelly extract. Sperm are obtained from commercially bred Xenopus laevis or Xenopus tropicalis.
- See the Table of Materials for specific items needed in the assay.
2. A two-chamber assay for frog sperm chemotaxis
- Anesthetize the frog by immersion in water containing 0.07% benzocaine, decapitate using a pair of carborundum edged scissors, and double pith to ensure euthanasia. Cut away abdominal skin to expose muscle. Make midline and lateral cuts in abdominal muscle and retract. Gently retract intestines and fat to reveal white, bean-shaped testes. Trim away connective tissue using a fine scissors being careful to avoid blood vessels. Remove a testis, wash away any blood using 1.5 x OR2 buffer, then roll the testis on filter paper to remove excess buffer and small adherent blood vessels, and then place the testis in a plastic pitre dish in 0.2 ml of 1.5 x OR2 buffer. Fill a 5 ml syringe with 2 ml of 1.5 x OR2 buffer, gently poke 10-20 holes in the testis over most of the surface at one end, insert the needle at the opposite end and gently inject buffer to flush out sperm. Alternatively, one can inject buffer while poking the exit holes to flush out sperm. Transfer the sperm suspension using a micropipette with a cut-off tip (avoiding shearing of the sperm) to a microcentrifuge tube and place on ice.
- Estimate the number of frog sperm obtained by diluting sperm 1:100 in 1.5 x OR-2 and taking a 20 μl sample of the mixed sperm suspension and counting the number of cells using a hemocytometer and the large 1 mm2 area. Using the hemocytometer count and sampling ratio calculate sperm density and the total number of sperm harvested. This total is usually 2 - 6 x 107 sperm in a volume of about 2 ml when both testes are used. Dilute the stock sperm suspension with 1.5 x OR2 buffer to obtain a sperm density of 2 x 107/ml. Use the sperm for assay within 2 to 3 hours of preparation. Assess sperm motility by diluting 5 μl of sperm 1:10 with F-1 buffer and visualizing movement using phase contrast optics. At least 40% to 50% of the sperm should be motile. Even suitable preparations will contain a large number of immotile sperm most of which are immature.
- Prepare a new 24-well plastic tissue culture plate by micropipetting 700 μl of F1 buffer into each well. Each well should be about 15 mm in diameter at the bottom.
- Start a series of assays by diluting 100 μl of sperm suspension with 900 μl of F1 buffer at room temperature (about 21 to 23°C) in a microcentrifuge tube to activate sperm motility by osmotic shock. Each time fresh sperm is activated in this manner, the sperm must be used within 1-2 minutes.
- Place a 12 μm porosity insert (12 mm o.d.) into a buffer-filled well; make sure the insert placement is off-center leaving a space to one side. Immediately transfer 400 μl of motility activated sperm into the well by a micropipette having a cut-off tip. Apply the sperm suspension to the wall of the filter insert and allow it to run down onto the filter at the bottom of the insert; the sperm suspension should not be pipetted directly onto the filter.
- Carefully micropipette 50 μl of chemoattractant agent into the well in the space between the well and the off-center filter insert. One must be careful to deposit the drop where the side and bottom of the well meet and withdraw the pipette with no disturbance to the system. Specifically, the plunger on the micropipette should be pushed only to the first stop so as to completely eject the sample but no air bubbles.
- Repeat steps 2.5 and 2.6 above to start as many assays as needed, then incubate the plate until the first assay started has incubated 50 minutes. Typically, one can start an assay every 45 to 60 seconds. Note that the assay can be streamlined by an experienced person or by two persons working together. In this case, one or two rows of assays can be initiated with a larger stock of motile sperm and faster pipetting providing that sperm are always used within 1 to 2 minutes of activation.
- Stop each assay in the sequence started. First, carefully steady the filter insert with one hand and with the other carefully remove most or all of the sperm suspension in the upper chamber by micropipette or by suction with a pasteur pipette. Immediately, using a fine tweezers, pull out the filter insert and discard. Care must be used at this step, least the remaining sperm be swept through the filter artifactually raising the values for sperm passage.
- From each plate well, transfer the entire sperm suspension to a microcentrifuge tube. It is important to mix the sperm suspension in the well before withdrawal since sperm tend to settle to the bottom. Add 15 μl of 25% formaldehyde to a final concentration of 0.5 % v/v and refrigerate if sperm counts are not done the same day.
- Pellet the sperm in each tube using a 10 second push button spin on a personal microcentrifuge having a maximum speed of 2000 x g. Remove all but 100 μl of supernatant from each tube, then resuspend the pellet in that volume. Take 20 μl of sperm suspension, dilute 1:10 with distilled water, and count sperm on a hemocytometer using a 40x objective on an upright microscope. Use the counts and dilution factors to calculate the total number of sperm that passed through the filter in each assay.
3. Frog sperm tracking assay using a Zigmond chamber
- Prepare the inverted microscope workstation for videotaping. We use a Nikon Elipse TE300 inverted microscope equipped with either a Sony DXC-390 3-chip color analogue video camera or a Hamamatsu ORCA-03G monochrome digital camera. The Zigmond chamber slide must rest on the stage up-side-down with a sufficient opening in the stage plate to accommodate a 22x40 mm cover glass. This arrangement is necessitated by the fact that frog sperm are unable to swim against gravity. Focus either a 4x or 10x objective lens on the observation platform running between the two troughs.
- Test to make sure that the camera is focused and centered on the observation platform. If the Sony camera is used, the output is sent to a video monitor and to an A/D converter (frame grabber) capable of digitizing 7 frames per second from the video signal. The digital stream is processed using a computer running at 3 GHz or greater preferably with 4 Gb or more of RAM. Although we use Scion Image software (a custom Windows version of NIH Image) controlling a Scion CG-7 frame grabber, these products are no longer available. A current solution is to use Image J software with a VirtualDub Plug-in for analogue video camera capture. If the Hamamatsu camera is used, its digital output is processed by Olympus cellSens software and displayed on the computer monitor. In both cases, the data is saved on either an internal or external hard drive as an 8-bit tiff stack. Use of Scion Image software requires conversion of the Tiff image sequence to a stack using Image J.
- The microscopic field should include most or all of the observation platform width. Use phase contrast or dark field optics for the best results. The distance between the observation platform surface and the bottom of the cover glass should be 15 to 20 μm as determined by differential focusing on each surface.
- Prepare the chemoattractant at an appropriate concentration in F1 buffer. Prepare Xenopus laevis sperm as described previously and store in 1.5 x OR2 buffer on ice until use.
- Assemble the Zigmond chamber. Start with a dry clean chamber. Using a micropipette place a line of silicone oil (4 μl) about 5 mm from and parallel to the outer edge of each trough. Place a 22x40 mm cover glass onto the chamber allowing the silicone oil to evenly spread to the outer edge of each trough. If preliminary experiments show that sperm sticking is a problem, one may need to coat the cover glass with nitrocellulose as suggested by Fabro et al.3. Alternatively, inclusion of protein in the buffers used (e.g. 1% BSA) can also reduce sperm sticking.
- Invert the chamber and place over the circular cutout in the microscope stage being careful that the cover glass does not make contact with the stage. This inverted configuration is necessary to bring Xenopus sperm from the trough onto the platform. Unlike mammalian sperm, Xenopus sperm are not strong enough to swim against gravity to reach the platform.
- Activate 20 μl of Xenopus sperm in 1.5 x OR2 buffer by mixing 1:10 with F1 buffer at room temperature. Using a micropipette with a cut tip, immediately transfer 70 μl of motility-activated sperm into the trough. This is achieved by holding the micropipette at a low angle and placing the tip at the side opening of the trough. The cell suspension ejected fills the trough and bridge by capillary action. Next, fill the opposite trough in the same manner using a chemoatttractant solution.
- Begin videotaping within 3 minutes (Xenopus sperm have a limited motility lifetime) and continue for 5 minutes. At the end of videotaping, disassemble the chamber and wash the troughs and observation platform with a pressurized stream of water and then ethanol from a squirt bottle. Remove silicone oil from the upper surfaces with paper wipes being careful not to contaminate the observation platform and troughs.
- If needed, convert the video data captured to a Tiff image sequence or stack using Image J software. Then open the file in Image J and in the first 21 frames (3 seconds) choose up to 50 sperm to be tracked. To avoid bias, chose sperm from all regions of the observation field and without knowledge of their trajectory data. Capture two-dimensional cell trajectories in the x-y plane as a function of time (xyt data sets) for each sperm by point and click of the mouse using the MtrackJ plug-in module for Image J. Visualize trajectories and calculate trajectory distances, axis components and velocities within MtrackJ. Alternatively, carry out these operations and further numerical analysis (e.g. directionality, chemotaxis parameters and parameter histograms) by importing xyt data sets into Microsoft Excel.
- Plot trajectories for each sperm in Excel to detect overall patterns of movement including linear, curvilinear, and circular patterns as well as features such as turns. Utilize xyt data sets to calculate average curvilinear velocity, net travel along the X (gradient)- and Y-axis, and orientation parameters such as the average angle of travel relative to the gradient axis.
4. Representative Results:
Important technical parameters in the two chamber assay are size and shape of the chamber, the porosity of the membrane, and the length of incubation. The size of the upper chamber holding the sperm should not be so large in diameter as to require a large volume of sperm (preferably 0.5 ml or less) nor should the upper chamber be so deep as to create a high column of cell suspension (<1 cm). The volume of lower chamber buffer surrounding the upper chamber insert should exactly match the level of the cell suspension in the insert so as not to create a transmembrane hydrostatic pressure that would artifactually force sperm through the membrane. Placement of the empty insert into the bottom well first, followed by sperm loading results in an initial upwards hydrostatic pressure that prevents sperm from going through the membrane during loading. Choice of membrane pore size is determined by the size of the sperm, and the commercial availability of porous membrane inserts. We find that assays with frog sperm can utilize either 8 or 12 μm diameter pores although 12 μm pore provide for passage of higher numbers of sperm allowing more accurate counting. Pore sizes greater than 12 μm do not appear to be commercially available. An alternative to using tissue culture inserts is the use of the Neuroprobe membranes designed for chemotaxis assays in 96-well plates. These offer a potentially greater throughput and a wider range of pore diameters. Although larger mammalian sperm would seem to require a larger pore diameter, we have successfully assayed mouse sperm chemotaxis using inserts with 12 μm pores (Burnett, unpublished observations). In contrast, smaller pore sizes might be adequate for smaller sperm (e.g. sea urchin) although we have not yet tested this possibility.
One disadvantage of the two-chamber assay described is that sperm are placed in the top chamber thus inevitably resulting in some sperm passage by gravity, thus decreasing the signal to background ratio. This is necessitated by the fact that Xenopus sperm are not vigourous enough in their motility to swim against gravity as are mammalian sperm. Another difficulty in assaying Xenopus sperm is the fact that their motility life time is short - 5 to 15 minutes after activation. This restriction is the basis for needing to activate a new batch of sperm every 2 to 3 minutes when carrying out multiple assays. As a result, time course studies have shown that most sperm passage occurs within the first 20 minutes of assay5. Although we use a 50 minute incubation period, this period could likely be shorted to 20 or 30 minutes. In contrast, for mouse sperm which remain motile for hours, we have used up to a 2 hour period of assay with good results (Burnett, unpublished observations).
In the two-chamber assay using frog sperm, the total number of sperm passing through the porous membrane is typically 10 to 20 thousand or about 1 to 2% of the sperm placed in the upper chamber insert. Presence of a chemoattractant gradient in the lower chamber can increase sperm passage as much as 4 to 10 fold. Figure 2 illustrates a typical dose response curve performed with this assay. An extract of Xenopus egg jelly ("egg water") containing the known chemoattractant protein allurin (red circles), was placed in the bottom chamber at a series of dilutions. The total egg water protein contained in each assay is given in micrograms/assay - the amount delivered in the original 50 μl volume. Since the protein delivered will form a diffusion gradient, the actual concentration range of protein that sperm respond to is not known but can be estimated to be 5 to 10 times lower than the protein concentration of the drop delivered. For this reason, we denote the amount of protein introduced, not the concentration. Usually we perform duplicate or triplicate assays for each dose and average the result; we then replicate the entire experiment 3 or 4 times using sperm from different males in each experiment. The mean and standard error of the mean is calculated for each dose with the standard error of the mean typically being 5 to 10% of the mean. Note that proteins without known chemoattractant activity such as bovine serum albumin (open circles, Fig. 2) may produce a low level, non-specific increase in sperm passage through the membrane. An interesting observation is that the dose response curve for egg water is multiphasic - a rising phase and a decreasing phase. This type of multiphasic relationship is common for sperm chemoattractants; the response of human sperm to follicular fluid shows a similar biphasic relationship6 that is thought be useful in that high concentrations of chemoattractant found in the vicinity of an egg may serve to diminish further searching responses on the part of the sperm.
Occasionally we find that the control values for this assay are higher than normal, thus reducing the fold increase produced by a chemoattractant. Usually, this can be traced to mechanical disruption of the filter insert during the assay. Thus, it is important that inserts are not disturbed while loading the sperm or chemoattractant, during the assay incubation or when the insert is removed. Particularly crucial is that the sperm reservoir in the insert is removed by micropipette or suction before lifting the insert out of the well. This ensures that sperm are not swept through the porous membrane into the bottom chamber as the insert is removed.
Important technical parameters in the Zigmond chamber assay include the distance between the coverglass and observation platform, the magnification of the video observation, the type of optics used, and the frame rate. Distance between the observation platform is typically 10 to 15 μm although this distance can be varied by the amount of silicone oil used to interface between cover glass and the chamber slide - the more oil, the greater the distance. A thin plane of fluid increases the amount of time needed for a gradient to form as well as the longevity of the gradient once formed. A thicker plane of fluid allows a more rapid gradient formation but the gradient has a shorter lifetime and less stability. The dynamics of gradient formation can be visualized by using a fluorescent dye or a fluorescent dextran in the chemoattractant well and using fluorescence microscopy to view the dynamics. The test reagent should be matched in molecular weight to the chemoattractant being used and the dynamics determined used to judge how many minutes should be allowed for gradient formation and recording of sperm movements.
The magnification used should be low overall (4x or 10x objective) if the entire observation platform is to be visualized as in the assay conditions we have described. On the other hand, higher magnification (40x or 63x objective) may be useful if one intends to monitor relatively short trajectory segments or wishes to resolve flagellar motions. Tracking can be carried out either by semi-manual methods as described in this paper or by automated tracking as practiced in more sophisticated software packages such as MetaMorph or Imaris Track. In either case, ease of tracking is very dependent on image contrast whether it be by operator or by software-assisted object recognition. Although bright field optics might be used in some cases, use of phase contrast optics or darkfield optics is commonly required. Finally, the frame rate depends on the time resolution desired in tracking. If semi-manual methods are used as in our procedure, one is probably limited to relatively low frame rate - 4 to 8 frames per second - due to the labor intensive nature of recording data. On the other hand, video rate observations may be required for rapid responses such as flagellar wave forms. Frequently, experimental goals are best served by doing slower frame rate experiments and faster frame rate experiments separately since one also wishes to alter other parameters such as magnification, optics, or digital image processing.
Typical results for the Zigmond chamber tracking assay consist of a set of trajectories like those seen for fifty frog sperm in the video clip of Movie 1. Against an image of the observation platform, the trajectories of individual sperm are traced in red for a control experiment (no chemoattractant gradient present). The two-dimensional cell trajectories in the x-y plane as a function of time for each sperm can be plotted and logged by MtrackJ and imported into Microsoft Excel. For trajectory analysis, we designate the chemoattractant gradient axis as the X-axis and the Y-axis as the orthogonal axis, consistent with the convention originally developed by Fabro et al.3. As shown in the diagram of Figure 3, the actual trajectory taken is composed of steps, the sum of whose length equals the curvilinear distance traveled in the trajectory (blue/gold arrows). The net distance and orientation of travel by each sperm is a vector connecting the first and last point of the trajectory (red diagonal arrow). The net travel can be separated into its X-axis component and Y-axis component (red dashed arrows; also termed net delta X and net delta Y, respectively).
Because chemotaxis in vertebrate sperm can involve relatively subtle shifts in direction of travel, large numbers of sperm (100 to 300), randomly selected, are usually analyzed for each condition often requiring pooled data from 4 to 6 independent experiments. For purposes of illustration, however, we will use data from only fifty frog sperm. Table 1 illustrates common parameters used to detect changes in sperm travel. The mean net travel along the X-axis will increase significantly if, in the presence of a chemoattractant, the sperm population as a whole follows trajectories that more closely align with the gradient. In our example, the mean net X-axis travel increased over three fold in the presence of egg water. One can also plot a histogram of net X-axis travel for the sperm population which has the advantage that smaller subpopulations of responsive sperm may be detected. Two parameters developed by Fabro et al.3 can also help detect such populations. Both the percentage of sperm showing positive net X-axis travel (% ΔX>0) and the percentage of sperm showing net linear travel no greater than 45 degrees from the gradient axis (%ΔX/|ΔY|> 1) can increase dramatically if the entire sperm population is sensitive to the chemoattractant or by smaller but still significant amounts if subpopulations of sperm are sensitive. Our example in Table 1 shows increases in both parameters for fifty frog sperm exposed to an egg water gradient. Note that random non-oriented movement will give non-zero values for both of these parameters of 50% and 25% respectively; control values higher than this (such as those in Table 1) represent a background due to either a low sample number or to a bias in sperm orientation particular to the experimental design.
Directionality of sperm travel in response to chemoattractant agents can be directly assessed by theta, the angle between the vector of net travel for each sperm and the gradient (X)-axis. Our example in Table 1 shows that the mean theta decreased for sperm swimming in an egg water gradient indicating that sperm trajectories as a population were better aligned with the gradient. Similar to net X-axis travel (see above), thetas for the sperm population can be expressed as a distribution, an approach also sensitive to subpopulations of responsive sperm and one that was recently studied by Gakamsky et al7.
Finally, curvilinear and instantaneous velocities for individual sperm can also be extracted from the data describing two-dimensional cell trajectories in the x-y plane as a function of time. One might find that there is an increase in velocity as well as a shift in orientation of travel, thus suggesting a chemokinetic response as well as a chemotactic response.
These data represent a starting point for analysis of sperm trajectories. Further analysis could include measurements of trajectory linearity and curvature on a moment by moment basis as carried out by Bohmer et al.8 and Shiba et al.9, the automated detection of turns as carried out by Burnett et al.10, and nonlinearity as gauged by fractal analysis11,12. Provided that sperm swimming these trajectories are monitored at higher magnification, one could also image flagellar motions and real time calcium signals as carried by several laboratories 8,9,13,14,15,16. Such studies have demonstrated that both invertebrate and mammalian sperm respond to chemoattractants by sharp turns of the sperm up gradient toward the chemoattractant source. These turns are accompanied by flagellar bends that alter sperm orientation much as a rudder would, bends that appear to initiated by defined, wave like calcium signals propagating through the flagellum. Thus, the ultimate goal of sperm tracking is to correlate signaling system dynamics with changes in flagellar propulsion that serve as the basis of sperm orientation in a chemotactic gradient. These goals have yet to be reached in Xenopus sperm which travel in a helical motion17, exhibit low curvature in their trajectories10, and whose calcium signals have yet to be monitored.
Although we have focused here on detailing the assay methods we use for Xenopus laevis sperm, both the two chamber sperm accumulation assay and the Zigmond chamber tracking assay can be used for mammalian sperm if certain modifications are made. Both assays can be carried out at 37 °C if desired, by use of a slide warmer and, in the Zigmond chamber assay, a microscope stage warmer. Typically, mammalian sperm will be isolated, capacitated and incubated using appropriate mammalian buffers and chemoattractants, but data analysis will be similar to what is described here for amphibian species. One further difference is that the Zigmond chamber is usually placed upright on the stage of an upright microscope since mammalian sperm, unlike Xenopus sperm, can swim up onto the observation platform. As yet untested, these two assays may see application to sperm of a number of species in future work.

Figure 1. Schematic diagram of the two-chamber assay. Sperm are placed in an insert the bottom of which is a polycarbonate filter with 12 μm pores. A chemoattractant solution is carefully pipetted into the well to initiate formation of a concentration gradient.
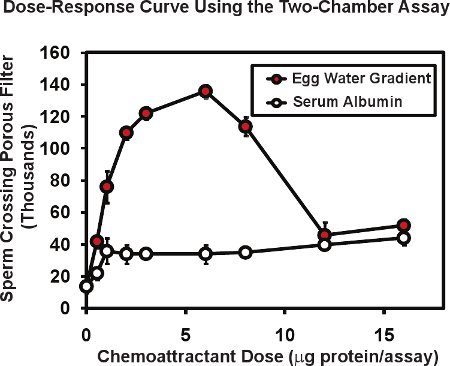
Figure 2. Representative data for frog sperm using the two-chamber sperm chemotaxis assay. Egg water prepared from X. laevis eggs exhibits a multiphase dose-activity curve that is characteristic of sperm chemoattractants. Bovine serum albumin increases sperm passage only slightly - a nonspecific effect of protein. Figure modified from Al-Anzi & Chandler5.
Movie 1. A video clip showing frog sperm trajectories plotted in red on the observation platform of a Zigmond chamber. The width of the platform is 1 mm. Click here to watch the video clip.
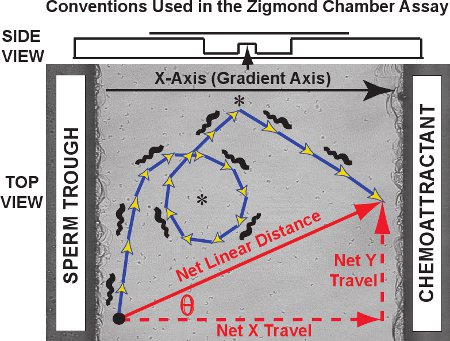
Figure 3. Axis conventions, a sperm curvilinear trajectory and a vector for net linear travel are plotted on the Zigmond chamber observation platform. Data obtained are of three types. First, the sperm trajectories themselves (blue/gold arrows) can reveal specific patterns such as circles and turns (asterisks). Curvilinear distance and curvilinear velocities can be measured for the trajectory path. Second, the net linear distance traveled over the entire trajectory, the net X (gradient) axis component of travel, the net Y axis component of travel and the angle theta between the X axis and the vector of net travel can be calculated for each trajectory and compared as a distribution over all sperm tracked. Third, instantaneous changes in velocity and direction of travel for segments within individual trajectories can be studied either individually or as a population. Above, a side view of the chamber is presented in a diagram.

Table 1. Parameters frequently used in analyzing data from the Zigmond chamber.
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Chemotaxis of cells moving by either amoeboid movement or flagella-powered swimming is found in many biological contexts and study of this phenomenon requires the availability of practical and reliable assays. Some examples of the phenomenon, such as attraction of sperm to a sea urchin egg or gathering of slime mold cells to form a fruiting body, have immediate visual impact. Quantitation of this phenomenon has been undertaken in a variety of ways as described by Eisenbach18. These assays include use of chemoa...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
No conflicts of interest declared.
Podziękowania
We thank the W.M. Keck Bioimaging Laboratory for use of their video microscopy work station. This study was supported by NSF grant IBN-0615435.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| 24-well plates | BD Biosciences | 35/1147 | |
| 12 mm outer diameter inserts with 12 μm pore membrane | EMD Millipore | PIXP01250 | We previously used Costar-Corning transwell plate #3403 -now discontinued |
| Zigmond chamber | Neuroprobe | Z02 | |
| Silicone oil | GE Healthcare | SF1154 | Equivalent to Dow Corning 550 Fluid |
| Image J software | Wayne Rasband, Research Services Branch, National Institute of Mental Health | Free download at http://rsbweb.nih.gov/ij | Java program that runs on Windows, Linux and Mac |
| MtrackJ software |  Biomedical Imaging Group Biomedical Imaging Group | Free download at http://www.imagescience.org/meijering/software/mtrackj/ | Java program that runs on Windows, Linux and Mac |
| Virtual Dub software |  GNU General Public Licensed GNU General Public Licensed | Free download via http://www.virtualdub.org/index.html | Setup instructions at the Image J website under plugins; for Windows only |
| cellSens software | Olympus Corporation | See website: http://www.olympusamerica.com/seg_section/product.asp?product=1070 | Controls and acquires images from a variety of cameras. Also has image processing capability |
Odniesienia
- Ward, G. E., Brokaw, C. J., Garbers, D. L., Vacquier, V. D. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J. Cell Biol. 101, 2324-2329 (1985).
- Olivera, R. G., Tomasi, L., Rovasio, R. A., Giojalas, L. C. Increased velocity and induction of chemotactic response in mouse spermatozoa by follicular and oviductal fluids. J. Reprod. Fertil. 115, 23-27 (1999).
- Fabro, G., Rovasio, R. A., Civalero, S., Frenkel, A., Caplan, S. R., Eisenbach, M., Giojalas, L. C. Chemotaxis of capacitated rabbit spermatozoa to follicular fluid revealed by a novel directionality-based assay. Biol. Reprod. 67, 1565-1571 (2002).
- Sugiyama, H., Burnett, L., Xiang, X., Olson, J., Willis, S., Miao, A., Akema, T., Bieber, A. L., Chandler, D. E. Purification and multimer formation of allurin, a sperm chemoattractant from Xenopus laevis egg jelly. Mol. Reprod. Dev. 76, 527-536 (2009).
- Al-Anzi, B., Chandler, D. Xenopus laevis egg jelly releases a sperm chemoattractant during spawning. Dev. Biol. 198, 366-375 (1998).
- Ralt, D., Goldenberg, M., Fetterolf, P., Thompson, D., Dor, J., Mashiach, S., Garbers, D. L., Eisenbach, M. Sperm attraction to a follicular factor(s) correlates with human egg fertilizability. Proc. Natl. Acad. Sci. U.S.A. 88, 2840-2844 (1991).
- Gakamsky, A., Schechtman, E., Caplan, S. R., Eisenbach, M. Analysis of chemotaxis when the fraction of responsive cells is small--application to mammalian sperm guidance. Int. J. Dev. Biol. 52, 481-487 (2008).
- Böhmer, M., Van, Q., Weyand, I., Hagen, V., Beyermann, M., Matsumoto, M., Hoshi, M., Hildebrand, E., Kaupp, U. B. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J. 24, 2741-2752 (2005).
- Shiba, K., Baba, S. A., Inoue, T., Yoshida, M. Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response. Proc. Natl. Acad. Sci. U.S.A. 105, 19312-19317 (2008).
- Burnett, L. A., Sugiyama, H., Bieber, A. L., Chandler, D. E. Egg jelly proteins stimulate directed motility in Xenopus laevis sperm. Mol. Reprod. Dev. 78, 450-462 (2011).
- Abaigar, T., Barbero, J., Holt, W. V. Trajectory variance and autocorrelations within single sperm tracks as population level descriptors of sperm track complexity, predictability and energy generating ability. J. Androl. , Forthcoming (2011).
- Mortimer, S. T., Swan, M. A., Mortimer, D. Fractal analysis of capacitating human spermatozoa. Hum. Reprod. 11, 1049-1054 (1996).
- Guerrero, A., Carneiro, J., Pimentel, A., Wood, C. D., Corkidi, G., Darszon, A. Strategies for locating the female gamete: the importance of measuring sperm trajectories in three spatial dimensions. Mol. Hum. Reprod. 17, 511-523 (2011).
- Yoshida, M., Yoshida, K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 17, 457-465 (2011).
- Spehr, M., Schwane, K., Riffell, J. A., Zimmer, R. K., Hatt, H. Odorant receptors and olfactory-like signaling mechanisms in mammalian sperm.Mol. Cell. Endocrinol. 250, 128-136 (2006).
- Veitinger, T., Riffell, J. R., Veitinger, S., Nascimento, J. M., Triller, A., Chandsawangbhuwana, C., Schwane, K., Geerts, A., Wunder, F., Berns, M. W., Neuhaus, E. M., Zimmer, R. K., Spehr, M., Hatt, H. Chemosensory Ca2+ dynamics correlate with diverse behavioral phenotypes in human sperm. J. Biol. Chem. 286, 17311-17325 (2011).
- Tholl, N., Naqvi, S., McLaughlin, E., Boyles, S., Bieber, A. L., Chandler, D. E. Swimming of Xenopus laevis sperm exhibits multiple gears and its duration is extended by egg jelly constituents. Biol. Bull. 220, 174-185 (2011).
- Eisenbach, M. Sperm chemotaxis. Rev. Reprod. 4, 56-66 (1999).
- Riffell, J. A., Zimmer, R. K. Sex and flow: the consequences of fluid shear for sperm-egg interactions. J. Exp. Biol. 210, 3644-3660 (2007).
- Corkidi, G., Taboada, B., Wood, C. D., Guerrero, A., Darszon, A. Tracking sperm in three-dimensions. Biochem. Biophys. Res. Commun. 373, 125-129 (2008).
- Himes, J. E., Riffell, J. A., Zimmer, C. A., Zimmer, R. K. Sperm chemotaxis as revealed with live and synthetic eggs. Biol Bull. 220, 1-5 (2011).
- Sun, F., Giojolas, L. C., Rovasio, R. A., Tur-Kaspa, I., Sanchez, R., Eisenbach, M. Lack of species-specificity in mammalian sperm chemotaxis. Dev. Biol. 255, 423-427 (2003).
- Guidobaldi, H. A., Teves, M. E., Uñates, D. R., Anastasía, A., Giojalas, L. C. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS One. 3, e3040-e3040 (2008).
- Oren-Benaroya, R., Orvieto, R., Gakamsky, A., Pinchasov, M., Eisenbach, M. The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum. Reprod. 23, 2339-2345 (2008).
- Teves, M. E., Guidobaldi, H. A., Uñates, D. R., Sanchez, R., Miska, W., Publicover, S. J., Morales Garcia, A. A., Giojalas, L. C. Molecular mechanism for human sperm chemotaxis mediated by progesterone. PLoS One. 4, 8211-82 (2009).
- Blengini, C. S., Teves, M. E., Uñates, D. R., Guidobaldi, H. A., Gatica, L. V., Giojalas, L. C. Human sperm pattern of movement during chemotactic re-orientation towards a progesterone source. Asian J. Androl. 13, 769-773 (2011).
- Strünker, T., Goodwin, N., Brenker, C., Kashikar, N. D., Weyand, I., Seifert, R., Kaupp, U. B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 471, 382-386 (2011).
- Lishko, P. V., Botchkina, I. L., Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 471, 387-391 (2011).
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 115, 453-465 (1962).
- Zigmond, S. H., Lauffenburger, D. A. Assays of leukocyte chemotaxis. Annu. Rev. Med. 37, 149-155 (1986).
- Villanueva-Diaz, C., Vadillo-Ortega, F., Kably-Ambe, A., Diaz-Pérez, M. A., Krivitzky, S. K. Evidence that human follicular fluid contains a chemoattractant for spermatozoa. Fertil. Steril. 54, 1180-1182 (1990).
- Villanueva-Diaz, C., Arizs-Martinez, J., Bermejo-Martinez, L., Vadillo-Ortega, F. Progesterone induces human sperm chemotaxis. Fertil. Steril. 64, 1183-1188 (1995).
- Olson, J., Xiang, X., Ziegert, T., Kittleson, A., Rawls, A., Bieber, A., Chandler, D. E. A. llurin a 21 kD sperm chemoattractant from Xenopus egg jelly, is homologous to mammalian sperm-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 98, 11205-11210 (2001).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone