Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
The Use of Cystometry in Small Rodents: A Study of Bladder Chemosensation
W tym Artykule
Podsumowanie
Cystometry is an efficient technique to measure bladder function of small animals in vivo. The bladder is continuously infused at rates controlled through an intravesical catheter, whereas the urethra is left free for micturition. This allows for repetitive filling and emptying of the bladder, while intravesical pressure and voided volume are recorded.
Streszczenie
The lower urinary tract (LUT) functions as a dynamic reservoir that is able to store urine and to efficiently expel it at a convenient time. While storing urine, however, the bladder is exposed for prolonged periods to waste products. By acting as a tight barrier, the epithelial lining of the LUT, the urothelium, avoids re-absorption of harmful substances. Moreover, noxious chemicals stimulate the bladder's nociceptive innervation and initiate voiding contractions that expel the bladder's contents. Interestingly, the bladder's sensitivity to noxious chemicals has been used successfully in clinical practice, by intravesically infusing the TRPV1 agonist capsaicin to treat neurogenic bladder overactivity1. This underscores the advantage of viewing the bladder as a chemosensory organ and prompts for further clinical research. However, ethical issues severely limit the possibilities to perform, in human subjects, the invasive measurements that are necessary to unravel the molecular bases of LUT clinical pharmacology. A way to overcome this limitation is the use of several animal models2. Here we describe the implementation of cystometry in mice and rats, a technique that allows measuring the intravesical pressure in conditions of controlled bladder perfusion.
After laparotomy, a catheter is implanted in the bladder dome and tunneled subcutaneously to the interscapular region. Then the bladder can be filled at a controlled rate, while the urethra is left free for micturition. During the repetitive cycles of filling and voiding, intravesical pressure can be measured via the implanted catheter. As such, the pressure changes can be quantified and analyzed. Moreover, simultaneous measurement of the voided volume allows distinguishing voiding contractions from non-voiding contractions3.
Importantly, due to the differences in micturition control between rodents and humans, cystometric measurements in these animals have only limited translational value4. Nevertheless, they are quite instrumental in the study of bladder pathophysiology and pharmacology in experimental pre-clinical settings. Recent research using this technique has revealed the key role of novel molecular players in the mechano- and chemo-sensory properties of the bladder.
Protokół
1. Laboratory Animals
- Animals (mice, rats) are housed in a specialized animal facility with a 12-hr light-dark cycle and ad libitum access to water and standard food pellets. Both age and sex of the animals are important parameters that should be standardized according to the needs. We typically perform cystometry in 10 - 12 week old female animals5,6.
- All animal experiments are carried out in accordance with the European Union Community Council guidelines and approved by the local ethics committee.
2. Anesthesia
- Isoflurane anesthesia is used for performing small surgical procedures, since it is easy to dose due to its short half-life. Appropriate waste gas scavenging is necessary when using isoflurane.
- Animals are placed in a closed box, gassed with isoflurane 5% in pure oxygen (1 l/min).
- After induction, anesthesia at the appropriate level is maintained by a small mask (a cut 10 ml syringe) by 0.8 - 1 l/min oxygen with 1 - 1.5% of isoflurane.
- For mice, urethane anesthesia is used during functional recordings (cfr. Infra). Urethane anesthesia is used during functional recordings because, in contrast to most other anesthetics, the micturition reflex is not suppressed by this anesthetic agent4. Urethane 1.2 g/kg body weight is administered subcutaneously (s.c.) 60 min before the start of the recordings. If necessary, the dose of urethane needed to obtain proper anesthesia can be titrated by administering additional doses (0.1 g/kg) subcutaneously. It should be noted, however, that additional anesthesia may alter the voiding efficiency and consequently the micturition frequency7,8. Thus, the cystometric recordings should be closely monitored and discarded whenever significant deviations from the control behavior are observed. Anesthetics should not be administered while test compounds are applied to avoid misinterpretation of the data.
3. Surgical Procedure - Bladder Catheter Implantation
- Surgical instruments are sterilized in an autoclave prior to use. Shave the abdomen of the animal, disinfect with ethanol 70% and perform a low midline laparotomy to expose the bladder.
- Under a surgical microscope, place a purse-string suture in the bladder dome using a non-absorbable, monofilament suture (size 6-0).
- Perform a small cystostomy (we preferentially use a 18 G needle) inside the purse string and insert a PE 50 polyethylene catheter, with a small cuff at the end (obtained by carefully heating the tubing) through this hole. In our hands, thinner tubes (PE 10) have a higher and more variable resistance. The tubing can be disinfected by putting it in an ethanol 70% solution or sterilized in an autoclave. Before implantation, the tubing should be flushed with saline.
- Secure the purse string around the tube with a surgeon's knot. Pull the catheter gently outwards until the bell-shaped tip is positioned right under the suture. Push a piece of 18 G catheter towards the suture to prevent leaking.
- Infuse a small volume of saline (100 - 200 μl) gently to check for leaks. If leaks are present, an additional suture should be placed.
- Close the abdominal muscles using monofilament sutures, leaving a passage for the catheter in the upper part of the incision.
- Tunnel the tube to the interscapular region using a hollow metal rod, thereby preventing the animals to bite the tube during the experiment.
- The skin is closed using non-absorbable, monofilament sutures. The implanted catheter is flushed with saline to check its permeability.
- Before the animals awaken, analgesics are given subcutaneously (buprenorphine, 0.05 mg/kg).
4. Setup and Cystometry
- Depending on the experimental question (and the species used) cystometry is performed in awake (restraint or unrestraint) or urethane-anesthetized animals. Whereas cystometry is perfectly feasible in conscious rats, which tend to remain calm in a restrained environment, we usually perform cystometry under urethane anesthesia (1.2 mg/kg, subcutaneously) when using mice3,5,6,9.
- In anesthetized mice, body temperature is constantly monitored and kept at 36.5 °C ± 0.2 °C by means of a temperature probe and a heating lamp.
- The set-up is calibrated before every experiment according to the manufacturers' instructions.
- The implanted catheter is connected by a luer stub to a 3-way tap, which is connected to the pressure transducer (Edwards Lifesciences) on one side and an infusion pump on the other (Harvard Apparatus). The pressure transducer is connected via an amplifier (78534c monitor, Hewlett Packard) to the data acquisition system (DI-730-USB, Dataq instruments) and a computer. Windaq/Lite software can be used for recording.
- The infusion pump is started, enabling the infusion of saline or PBS at, for example, 100 μl/min (in rats) or 20 μl/min (in mice). As such the bladder will be filled at a constant rate, while the intravesical pressure is recorded.
- Figure 5 depicts a typical pressure trace: during fluid infusion, there is a slow pressure buildup in the bladder, until a certain volume/pressure is reached, the micturition threshold. Once this threshold is reached, the bladder will contract and subsequently the urinary sphincter will open, allowing the passage of urine through the urethra. As such, the bladder is emptied, the contraction will stop and the pressure will drop again to the "basal" level.
- As the rat is placed in a metabolic cage and above a digital balance, the voided volume can be measured simultaneously, giving information about the voided volume. Due to the small voided volumes in mice, it can be very challenging using this system. Therefore, voided volume is determined using small filter papers weighed before and after voiding.
- Typically, we let the system equilibrate for 30 min, followed by a recording period of 30 min. Then, drugs can be administered systemically or intravesically and another 30 min are recorded.
- Recorded data can be exported as .csv files that can be imported into a specialized software for quantitative analysis. We typically use Origin (OriginLab Corporation, MA, USA).
- After experiments, animals are sacrificed by cervical dislocation (mice) or CO2 intoxication (rats).
5. Representative Results

Figure 1. Laparotomy overview. A) Place the rat in the supine position. B) Shave and antiseptically prepare the surgical site. C) Incision of the skin. D) Incision of the abdominal muscles, and bladder exposure.

Figure 2. A purse-string suture.
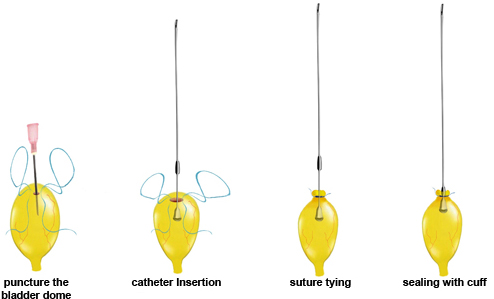
Figure 3. Catheter implantation.
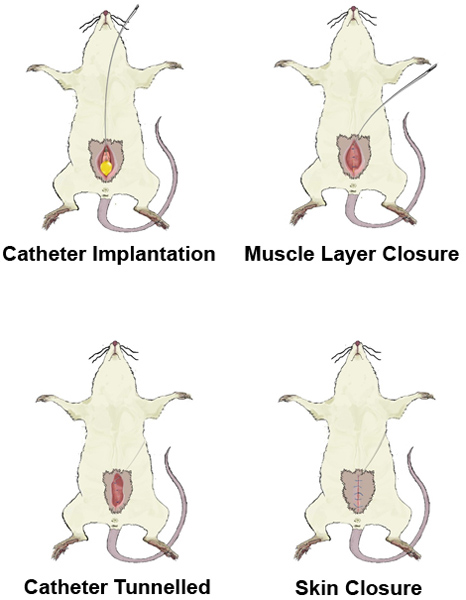
Figure 4. Tunnelling of the catheter.
Examples of pressure measurements obtained during intravesical perfusion of saline in a conscious rat and an anesthetized mouse are shown in Figure 5. Multiple parameters can be extracted from the pressure signal (e.g. the intercontractile interval, the baseline pressure and the threshold pressure). For comprehensive descriptions of these parameters, please see Andersson et al. (ref 4) and Yoshiyama et al. (ref 10).
We have recently used cystometry to identify the molecular targets of mustard oil (MO), a highly reactive compound that has been long used in experimental models of inflammation and hyperalgesia of visceral organs such as the urinary bladder11,12. Intravesical infusion of 10 mM MO induced a strong increase in the voiding frequency (decrease in the intercontractile interval) in wild type mice (Figure 6A, B) and a decrease of the voided volume6. Interestingly, MO induced similar changes in mice deficient of the MO receptor TRPA1. In contrast, MO induced much weaker changes in cystometric parameters in Trpv1 KO mice than in WT mice and was without any effect in Trpa1/Trpv1 KO mice. Together with measurements of the release of the Calcitonin Gene Related Peptide (CGRP)6, these data indicate demonstrate that TRPV1 may play a key role in visceral irritation induced by MO.

Figure 5. Representative traces of intravesical pressure recorded in a conscious female rat (A) and in an anesthetized female mouse (B). The lowest pressure is defined as the "baseline pressure" (red arrows). The pressure at the end of the filling phase is marked with blue arrows. The volume of fluid infused between these points, divided by the pressure difference, allows calculation of the compliance of the bladder wall (compliance = infused volume/(threshold pressure - baseline pressure). The "intercontractile interval" (ICI) is the time between two voiding contractions.
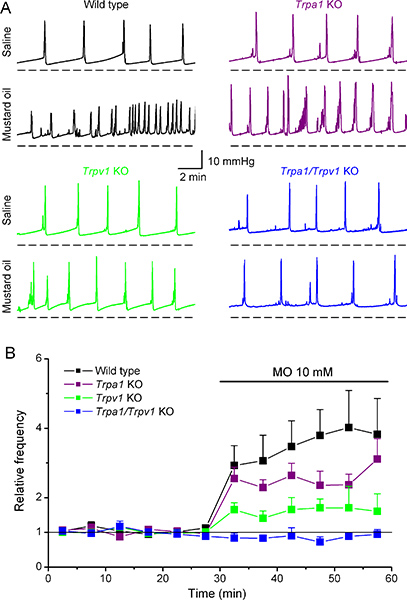
Figure 6. Effects of intravesical application of mustard oil on the cystometry pattern in wild type and Trpa1, Trpv1 and Trpa1/Trpv1 knockout mice. (A) Representative examples of intravesical pressure changes recorded in WT, Trpa1 KO, Trpv1 KO and Trpa1/Trpv1 KO mice in response to infusion of saline and 10 mM MO. (B) Time course of the average instantaneous voiding frequency before and during intravesical infusion of MO. For all mice, the data were normalized to the average frequency obtained during saline infusion. These data is adapted from Everaerts et al. (ref 6), with permission from Elsevier.
Dyskusje
The cystometry technique presented here allows performing in vivo measurements of bladder function in animal models. Rats are probably the most used animal model. Mice are more difficult to handle, but offer the advantage of using genetically manipulated animals. Because of the technical difficulty of using conscious mice, which tend to be very active resulting in loosening of the implanted catheter and changes in the intra-abdominal pressures that may influence the intravesical pressure, we advise to kee...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported by grants from the Belgian Federal Government (IUAP P6/28), the Research Foundation-Flanders (F.W.O.) (G.0565.07 and G.0686.09), the Astellas European Foundation Award 2009 and the Research Council of the KU Leuven (GOA 2009/07, EF/95/010 and PFV/10/006). P.U. and W.E. are doctoral fellows of the Research Foundation-Flanders (FWO). M.B. is a Marie Curie fellow. D.D.R. a fundamental-clinical fellow of the FWO.
Materiały
| Name | Company | Catalog Number | Comments |
| urethane | Urethane, Sigma-Aldrich | 315419 | group 2B carcinogen |
| isoflurane | Isoba, Schering-Plough Animal Health | ||
| polyethylene catheter | Intramedic Polyethylene tubing PE50, Becton Dickinson | 427411 | |
| surgical microscope | Op-Mi 6, Carl Zeiss | Op-Mi 6 | |
| purse-string suture | Prolene 6/0, Ethicon | 8610H | |
| fascia and skin suture | Ethilon 4/0 or 5/0, Ethicon | 662G or 661G | |
| postoperative analgesics | Temgesic, Schering-Plough Animal Health | dosage for rats: 0.05 mg/kg | |
| amplifier | 78534c monitor, Hewlett Packard | ||
| analytical balances and balance data acquisition software | FZ 300i, A&D | FZ-300i | |
| infusion pumps | pump 33, Harvard apparatus | HA33 | |
| cystometry recording system | Dataq instruments, DI-730 series and Windaq/Lite | DI-730-USB Windaq/Lite | |
| temperature registration | Fluke 52 KJ thermometer | 52 KJ | |
| pressure transducers | Edwards Lifesciences, pressure monitoring set | T322247A |
Odniesienia
- Everaerts, W., Gevaert, T., Nilius, B., De Ridder, D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol. Urodyn. 27, 264-2673 (2008).
- Fry, C. H. Animal models and their use in understanding lower urinary tract dysfunction. Neurourol. Urodyn. 29, 603-608 (2010).
- Gevaert, T. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J. Clin. Invest. 117, 3453-3462 (2007).
- Andersson, K. E., Soler, R., Fullhase, C. Rodent models for urodynamic investigation. Neurourol. Urodyn. 30, 636-646 (2011).
- Everaerts, W. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl. Acad. Sci. U.S.A. 107, 19084-19089 (2010).
- Everaerts, W. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr. Biol. 21, 316-321 (2011).
- Yoshiyama, M., Roppolo, J. R., Thor, K. B., de Groat, W. C. Effects of LY274614, a competitive NMDA receptor antagonist, on the micturition reflex in the urethane-anaesthetized rat. Br. J. Pharmacol. 110, 77-86 (1993).
- Yoshiyama, M., Roppolo, J. R., de Groat, W. C. Effects of MK-801 on the micturition reflex in the rat--possible sites of action. J. Pharmacol. Exp. Ther. 265, 844-850 (1993).
- Boudes, M. Functional Characterization of a Chronic Cyclophosphamide-Induced Overactive Bladder Model in mice. Neurourol. Urodyn. , (2011).
- Yoshiyama, M. Sex-related differences in activity of lower urinary tract in response to intravesical acid irritation in decerebrate unanesthetized mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R954-R960 (2008).
- McMahon, S. B., Abel, C. A model for the study of visceral pain states: chronic inflammation of the chronic decerebrate rat urinary bladder by irritant chemicals. Pain. 28, 109-127 (1987).
- Du, S., Araki, I., Yoshiyama, M., Nomura, T., Takeda, M. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology. 70, 826-831 (2007).
- Streng, T., Santti, R., Talo, A. Similarities and differences in female and male rat voiding. Neurourol. Urodyn. 21, 136-141 (2002).
- Igawa, Y. Cystometric findings in mice lacking muscarinic M2 or M3 receptors. J. Urol. 172, 2460-2464 (2004).
- Schroder, A., Newgreen, D., Andersson, K. E. Detrusor responses to prostaglandin E2 and bladder outlet obstruction in wild-type and Ep1 receptor knockout mice. J. Urol. 172, 1166-1170 (2004).
- Chen, Q. Function of the lower urinary tract in mice lacking alpha1d-adrenoceptor. J. Urol. 174, 370-374 (2005).
- May, V., Vizzard, M. A. Bladder dysfunction and altered somatic sensitivity in PACAP-/- mice. J. Urol. 183, 772-779 (2010).
- Thorneloe, K. S., Meredith, A. L., Knorn, A. M., Aldrich, R. W., Nelson, M. T. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am. J. Physiol. Renal. Physiol. 289, 604-610 (2005).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone