Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Engineering a Bilayered Hydrogel to Control ASC Differentiation
W tym Artykule
Podsumowanie
This protocol focuses on utilizing the inherent ability of stem cells to take cue from their surrounding extracellular matrix and be induced to differentiate into multiple phenotypes. This methods manuscript extends our description and characterization of a model utilizing a bilayered hydrogel, composed of PEG-fibrin and collagen, to simultaneously co-differentiate adipose-derived stem cells1.
Streszczenie
Natural polymers over the years have gained more importance because of their host biocompatibility and ability to interact with cells in vitro and in vivo. An area of research that holds promise in regenerative medicine is the combinatorial use of novel biomaterials and stem cells. A fundamental strategy in the field of tissue engineering is the use of three-dimensional scaffold (e.g., decellularized extracellular matrix, hydrogels, micro/nano particles) for directing cell function. This technology has evolved from the discovery that cells need a substrate upon which they can adhere, proliferate, and express their differentiated cellular phenotype and function 2-3. More recently, it has also been determined that cells not only use these substrates for adherence, but also interact and take cues from the matrix substrate (e.g., extracellular matrix, ECM)4. Therefore, the cells and scaffolds have a reciprocal connection that serves to control tissue development, organization, and ultimate function. Adipose-derived stem cells (ASCs) are mesenchymal, non-hematopoetic stem cells present in adipose tissue that can exhibit multi-lineage differentiation and serve as a readily available source of cells (i.e. pre-vascular endothelia and pericytes). Our hypothesis is that adipose-derived stem cells can be directed toward differing phenotypes simultaneously by simply co-culturing them in bilayered matrices1. Our laboratory is focused on dermal wound healing. To this end, we created a single composite matrix from the natural biomaterials, fibrin, collagen, and chitosan that can mimic the characteristics and functions of a dermal-specific wound healing ECM environment.
Protokół
1. Isolating Adipose-Derived Stem Cells (ASCs) 1, 5
Note: All procedures were performed at room temperature unless otherwise noted.
- Isolate rat perirenal and epididymal adipose and wash with sterile Hank's buffered salt solution (HBSS) containing 1% fetal bovine serum (FBS) as previously described 5. This study has been conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations and in accordance with the principles of the Guide for the Care and Use of Laboratory Animals.
- Mince the tissue and transfer 1-2 g into 25 mL of HBSS containing 1% FBS into a 50-mL tube and centrifuge at 500 g for 8 min at room temperature.
- Collect the free-floating adipose tissue layer and transfer to 125-mL Erlenmeyer flask and treat with 25 mL of collagenase type II (200 U/mL) in HBSS for 45 min at 37 °C on an orbital shaker (125 rpm).
- Carefully remove the liquid fraction (below oil and adipose layer) by pipeting and filter it sequentially through a 100- μm and 70- μm nylon mesh filter. Centrifuge the filtrate at 500 g for 10 min at room temperature, aspirate the supernatant, and wash the pellet twice with 25 mL of HBSS.
- Resuspend the cell pellet in 50 mL of growth medium (MesenPRO RS Basal Medium) supplemented with MesenPRO RS Growth Supplement, antibiotic-antimycotic (100 U/mL of penicillin G, 100 μg/mL of streptomycin sulfate, and 0.25 μg/mL of amphotericin B), and 2 mM of L-glutamine and pipette cells into two T75 flasks (25 mL/flask).
- Culture the ASC in a 5% CO2-humidified incubator at 37 °C (passage 2-4 ASCs are used for all experiments).
2. Preparing Chitosan Microspheres (CSMs)
Note: All procedures were performed at room temperature unless otherwise noted.
- CSMs are prepared by a water-in-oil emulsification process along with an ionic coacervation technique using our previous protocol 5. Emulsify an aqueous solution of chitosan (6 mL of 3%w/v chitosan in 0.5 M of acetic acid) in a 100 mL of an oil phase mixture consisting of soya oil, n-octanol (1:2 v/v) and 5% sorbitan-mono-oleate (span 80) emulsifier, using overhead (1700 rpm) and magnetic stirring (1000 rpm) simultaneously in opposite directions. This dual method of mixing ensures that micelles formed early on before cross-linking occurs can remain in solution and do not to settle to the bottom. Furthermore, the magnetic stir bar aids in de-aggregating chitosan during micelle formation and rigidization.
- Stir the mixture continuously stirred for approximately 1 hour until a stable water-in-oil emulsion is obtained. Initiate ionic cross linking with the addition of 1.5 mL of 1% w/v potassium hydroxide in n-octanol every 15 min for 4 h (24 mL total)
- After completion of the cross linking reaction, slowly decant the oil phase of the mixture containing CSM and immediately add the 100 mL of acetone, the solution will become cloudy, due to the oil residue. After 5 minutes, decant, add an additional 100 mL of acetone and incubate overnight at room temperature. The next day, re-wash the beads with acetone for five minutes. After performing these serial washes the solution should have turned clear, if not continue washing until all oil is removed and the acetone solution is clear.
- Dry the recovered spheres in a vacuum desiccator and analyze without further processing. You can determine the average CSM particle size, surface area per milligram, and the unit cubic volume by using a particle size analyzer.
- For subsequent experiments, wash the CSM three times with sterile water to remove residual salts and sterilize by washing overnight with 5 mL of absolute ethanol.
3. Determining the Number of Free Amino Groups in CSMs
Note: All procedures were performed at room temperature unless otherwise noted.
- Determine the number of free amino groups present in CSMs after ionic cross linking by using the trinitro benzenesulfonic (TNBS) acid assay of Bubnis and Ofner6. Incubate 5 mg of microspheres with 1 mL of 0.5% TNBS solution in a 50-mL glass tube for 4 h at 40 °C and hydrolyze with the addition of 3 mL of 6N HCl at 60 °C for 2 h.
- Cool the samples to room temperature and extract the free TNBS by adding 5 mL of deionized water and 10 mL of ethyl ether.
- Warm a 5-mL aliquot of the aqueous phase to 40 °C in a water bath for 15 min to evaporate any residual ether, cool to room temperature, and dilute with 15 mL of water.
- Measure the absorbance at 345 nm with a spectrophotometer using TNBS solution without chitosan as blank and the chitosan used for CSM preparation to determine the total number of amino groups. Estimate the number of free amino groups of the CSM relative to chitosan.
4. Loading ASC in CSM
Note: All procedures were performed at room temperature unless otherwise noted.
- Equilibrate 5 mg of sterilized CSMs from section 2.5 in sterile HBSS overnight and add to an 8- μm pore size membrane culture plate insert (24-well plate).
- After the CSMs have settled onto the membrane, carefully aspirate the HBSS and add 300 μL of growth medium to the inside of the insert and 700 μL of growth media to the outside of the insert.
- Resuspend ASCs at the appropriate concentration (1 × 104 to 4 × 104) in 200 μL of growth medium and seed over the CSM inside the culture plate insert. The final volume of medium within the culture insert, after seeding, is 500 μL.
- Incubate the ASC seeded on CSMs for 24 h in a 5% CO2 humidified incubator at 37 °C.
5. Determining the Percentage of ASC Loading and Cell Viability in CSMs
Note: All procedures were performed at room temperature unless otherwise noted.
- After incubation, pipet the ASC-loaded CSM in a sterile 1.5-mL microcentrifuge tube without disturbing the cells that have migrated into the insert membrane.
- Remove the residual medium and add 250 μL of fresh growth medium to the tube.
- To each tube, add 25 μL of MTT [3-(4,5-dimethylthiozole-2-yl)-2,5-diphenyltetrazolium bromide] solution (5 mg/mL) and incubate for 4 h in a 5% CO2 humidified incubator at 37 °C.
- After incubation, remove the medium, add 250 μL of dimethyl sulfoxide, and vortex the mixture for 2-5 min to solubilize the formazan complex.
- Centrifuge the CSM at 2700 g for 5 min, pipet off the supernatant and determine its wavelength absorbance at 570 nm and 630 nm using a standard plate reader, according to MTT manufacturer's specifications.
- Determine the cell number associated with the CSMs relative to the values obtained from defined ASC numbers cultured to develop a standard curve.
6. Preparation and Characterization of ASC-CSM Embedded in PEG-fibrin Gels
Note: All procedures were performed at room temperature unless otherwise noted.
- Polyethylene glycol (PEG) fibrin (PEG-fibrin) hydrogel prepared by Suggs et al.7 by dissolving the succinimidyl glutarate modified polyethylene glycol (PEG; 3400 Da) using 4 mL of tris-buffered saline (TBS, pH 7.8) and filter sterilize with a 0.22-μm filter just before starting the experiment. Dissolved PEG is only effective in this application for the first 3-4 hours.
- Mix 500 μl of fibrinogen stock (40 mg/mL in TBS, pH 7.8) and 250μl of PEG stock in a culture well of a 6-well plate and incubate for 20 minutes in a 5% CO2 humidified incubator at 37 °C. This mixture constitutes a molar concentration ratio of 1:10, SG-PEG-SG: fibrinogen.
- Take 250 μl of ASC-CSM at a concentration of 5 mg of CSM, (≈ 2 × 104 cells) and mix with the PEGylated fibrinogen solution.
- Immediately add 1 ml of thrombin stock (25U/mL) and quickly triturate once or twice with the pipette. After mixing the thrombin with the PEG-fibrinogen, immediately place the cell-gel mixture in a 12-well plate and incubated in a 5% CO2 humidified incubator at 37 °C for 10 min to allow for complete gelation. Since the gelation time is fast, do not try and hold the gel solution within the pipette tip for more than 5 seconds. The PEGylated fibrinogen is cleaved by thrombin and forms a PEGylated fibrin hydrogel. As such, the final gel product is referred to as PEG-fibrin.
- Wash the PEG-fibrin gels twice with HBSS and incubate with alpha minimal essential media (α-MEM) supplemented with 10% FBS in a 5% CO2 humidified incubator at 37 °C.
- Observe the migration of cells from CSM into the gel over an 11-day period using standard light microscopy techniques.
7. Preparation and Characterization of ASC-CSM Embedded in Collagen Gels
Note: All procedures were performed at room temperature unless otherwise noted.
- Mix ASC-CSM (5 mg containing ≈ 2 × 104 cells) with type 1 collagen (7.5 mg/mL) extracted from rat tail tendons according to the method of Bornstein8 and fibrillate after adjusting the pH to 6.8 using 2N NaOH.
- Add the fibrillated collagen-ASC-CSM mixture to a 12-well plate and incubate for 30 min in a 5% CO2 humidified incubator at 37 °C.
- After complete fibrillation, incubate the collagen-ASC-CSM gels for up to 11 days in a 5% CO2 humidified incubator at 37 °C.
- Observe the migration of cells from CSM into the gel over an 11-day period using standard microscopy techniques.
8. Development of Bilayered PEG-fibrin-(ASC-CSM)-Collagen Gel Constructs
Note: All procedures were performed at room temperature unless otherwise noted.
- To develop the bilayer construct, prepare both the collagen and the PEG-fibrin gels as described above, with slight modifications. Briefly, to study the migratory and co-induction properties of a single source of stem cells using two bioscaffolds, "sandwich" the ASC-CSMs between the collagen and the PEG-fibrin scaffolds by using a four-step process: 1) Load ASC onto CSM, 2) cast fibrillated collagen gel and layer the ASC-CSM beads over the collagen gel, 3) cast PEG-fibrin gel over the ASC-CSM-collagen gel and allow gel to solidify, and 4) add medium to well and insert to study cells in vitro or remove from insert for in vivo applications (see Figure 1).
- Prepare a 1-mL type 1 collagen (7.5 mg/mL) mixture as described above in 7.1, without adding ASC-CSM to the mixture. Place the mixture in a 6-well tissue culture insert (8-μm pore size) and incubate for 30 min in a 5% CO2 humidified incubator at 37 °C.
- After complete fibrillation, layer over the collagen surface 5 mg of ASC-CSM (10, 000 cells/mg) suspended in culture media (200 μl). After the microspheres have settled over the gel, prepare the PEG-fibrin gel as described in section 6.0, without adding ASC-CSM to the mixture, and layer the PEGylated fibrinogen/thrombin solution over the ASC-CSM- collagen layers. When preparing the PEG-fibrin gel, use 250 μl of cell culture medium in place of the 250 μl of medium containing the cells.
- Once completed, incubate the constructs for 30 min in a 5% CO2 humidified incubator to achieve complete gelation before feeding the construct with culture medium.
- After complete gelation, place 1 mL of medium in the upper chamber over the construct and 3 mL of medium in the lower chamber.
9. Making Stock Solutions
Note: All procedures were performed at room temperature unless otherwise noted.
- Calcium chloride stock (40 mM): Use only CaCl2.2H2O. Dissolve 588.4 mg of CaCl2.2H2O with 100 ml of deionized water. Sterilize using a 0.22-μm filter.
- Polyethylene glycol: PEG is highly reactive with oxygen and can become oxidized when exposed to room air. As such, PEG should be stored under nitrogen (N2) atmosphere. Accurately weigh 32 mg in a 2-ml centrifuge tube (make sure to purge the tubes with N2 before use) and store at -80 °C until ready for use. Dissolve 32 mg of PEG in 4 mL of TBS solution before use.
The following solutions must be made fresh before every experiment.
- TBS solution: (pH 7.75-7.77 at 25 °C; pH at 25 °C is very critical). To prepare 15 ml of TBS solution, carefully dissolve one buffer tablet in filter-sterilized deionized water and adjust to the required pH. Do not store the stock solution—prepare it fresh every time.
- Fibrinogen solution: (40 mg/ml). Dissolve fibrinogen powder in TBS to make the fibrinogen concentration 40 mg/ml. Dissolve it overnight using a magnetic stirrer at 4 °C. It is easier to dissolve the entire 1-g quantity with the required amount of TBS. The following day, remove the fibrinogen from 4 °C (usually cloudy) and allow it to warm in a water bath until a homogenous solution is obtained. Finally, filter sterilize the fibrinogen using a 0.45-μm filter.
- Thrombin solution (25 Units/ml): To make the thrombin solution, weigh out the required amount and dissolve with the prepared 40 mM CaCl2.2H2O. It's always a good practice to use the entire vial of thrombin to make the stock. Example: Dissolve a bottle of 5 kU thrombin with 200 mM of CaCl2.2H2O, aliquot and store at -20 °C.
10. Representative Results
The overall goal of the technique presented here is to demonstrate the potential of simultaneous matrix-driven differentiation of ASC into multiple phenotypes using CSM as a delivery vehicle. We demonstrate an in vitro strategy to deliver stem cells from CSMs into a bilayered collagen-PEG-fibrin scaffold. Characterization of ASC embedded within this scaffold revealed that ASC-loaded CSMs can be "sandwiched" in between a layer of collagen and PEG-fibrin simultaneously and differentially take cue from both extracellular environments to thrive under their new conditions. We first characterized the ability for the model system to maintain cell viability and migratory capacities. Collagen supported the ability of ASCs to maintain their "stemness," as was demonstrated by their expression of Stro-1 and their fibroblast-like morphology (Figure 2D and 2F). In contrast, PEG-fibrin induced the ASCs to differentiate toward a vascular phenotype, as is demonstrated by their tube-like structure morphology, their endothelial cell-specific expression of von Willebrand factor (Figure 2E and 2G), and pericyte-specific expression of NG2 and platelet-derived growth factor receptor beta (PDGFRβ) (data not shown). Furthermore, these observed phenotypes appeared to occur early in culture and were maintained over 11 days, as is demonstrated in Figure 3.
Tables and Figures
Benefits of Bilayer Construct:
- The scaffold alone without cells can perform as a bioactive scaffold.
- PEG-fibrin can induce stem cells to differentiate without the addition of growth factors.
- Collagen can assist in maintaining the stem cell phenotype of ASCs.
- A bilayer construct can be used as an active substrate for other cell types to migrate and proliferate (e.g., endothelial cells, fibroblast, keratinocytes, smooth muscle cells, pericytes).
- It can be used with hard-tissue engineering scaffolds like hydroxyaptite or demineralized bone to regenerate hard and soft tissue.
- It can be used to develop a multi-layered, diverse tissue-engineered construct (like dermal-vascular, vascular-epithelial, dermal-vascular-hypodermal, etc.).
- It uses a single-cell source to simultaneously develop multicellular compartments.
- It has the potential to integrate with the host tissue since it is of natural origin.
- CSM within the gel construct provides a platform for cells to migrate from.
- Chitosan, used to prepare CSM, is a well-known active chemo-attractant.
- The overall concept applied in this protocol of "matrix-driven stem cell differentiation" may be applied to other stem cells types. However, further investigation is warranted to determine the feasibility of matrix-driven differentiation. The bilayered gel scaffold can act as a reservoir to deliver the cells in a sustained and controlled manner.
- After reconstruction, the gels can still be separated into individual components.
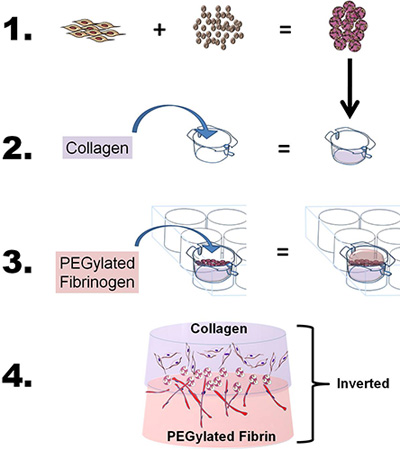
Figure 1. Schematic depicting the overall goal and process of the technique. 1) Adipose-derived stem cells (ASCs) are loaded onto chitosan microspheres. 2) Collagen is then poured into a 6-well insert, the pH adjusted to fibrillate the collagen, and the insert placed into a 6-well plate chamber. The ASC-loaded CSM spheres are then layered over the collagen. 3) The PEGylated fibrinogen is then poured over the collagen (ASC-CSM) and gelled by the addition of thrombin. 4) The final bilayer construct can then be removed from the culture insert and used for in vitro or in vivo analysis.
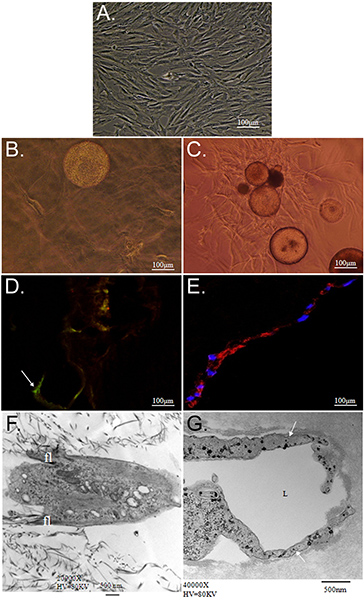
Figure 2. Characterization of ASC cultured within collagen and PEG-fibrin 3D matrices. A) Phase-contrast photomicrograph of isolated ASC passaged and maintained using routine 2-dimensional cell culture techniques. Photomicrographs B, D, and F depict ASC-CSM cultured within a 3-dimensional collagen gel; whereas C, E, and G show ASC-CSM cultured within a 3-dimensional PEG-fibrin gel, both at day 12. In B and C), ASCs are shown migrating away from the CSM sphere in both scaffold types. ASCs appear to have a flattened, spindle-like morphology in collagen (B), while maintaining their expression of the stem cell marker Stro-1 (D; arrow). When cultured in PEG-fibrin ASCs exhibit more tube-like structures and are induced to express such vascular cell markers as von Willebrand Factor (E). Transmission electron microscopy depicts the typical morphology demonstrated by ASCs within each scaffold. ASCs in collagen gel appear to have smaller filopodia (fl) extending from the body of the cell (F), whereas ASCs typically formed lumenal (labeled L) structures (G; arrow).
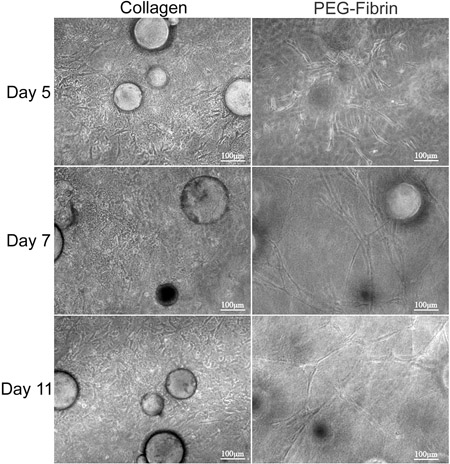
Figure 3. Morphological analysis of ASC-CSMs between bilayers of collagen and PEG-fibrin gels. ASC-CSMs were "sandwiched" between collagen and PEG-fibrin gels and maintained in culture for 11 days. The left column depicts ASCs migrating and proliferating within the collagen matrix and appear to take on a spindle-like morphology. The right column depicts ASCs migrating away from the CSMs and forming tube-like structures throughout the PEG-fibrin gel.
Dyskusje
ASCs are well-known for their ease of isolation and ability to differentiate toward various cell types. With the techniques described in this manuscript, we are able to exploit the plasticity of ASCs by exposing these cells to multiple biomatrices simultaneously. As cells migrate away from their CSM base and enter their surrounding extracellular environment, the cells take cue from the scaffold and can either maintain "stemness" (collagen) or be induced to differentiate toward vascular- and vascular-supportive cell types...
Ujawnienia
No competing financial interests exist.
Disclaimers
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the U.S. Government. The authors are employees of the U.S. Government, and this work was prepared as part of their official duties. All work was supported by the U.S. Army Medical Research and Materiel Command. This study was conducted under a protocol reviewed and approved by the U.S. Army Medical Research and Materiel Command Institutional Review Board and in accordance with the approved protocol.
Podziękowania
S.N. was supported by a Postdoctoral Fellowship Grant from the Pittsburgh Tissue Engineering Initiative. D.O.Z. is supported by a grant awarded from The Geneva Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| Hanks Balanced Salt Solution (HBSS) | GIBCO, by Life Technologies | 14175 | Consumable |
| Fetal Bovine Serum | Hyclone | SH30071.03 | Consumable |
| Collagenase Type II | Sigma-Aldrich | C6685 | Consumable |
| 70-μm Nylon Mesh Filter | BD Biosciences | 352350 | Consumable |
| 100-μm Nylon Mesh Filter | BD Biosciences | 352360 | Consumable |
| MesenPRO Growth Medium System | Invitrogen | 12746-012 | Consumable |
| L-Glutamine | GIBCO, by Life Technologies | 25030 | Consumable |
| CaCl2.2H2O | Sigma-Aldrich | C8106 | Consumable |
| T75 Tissue Culture Flask | BD Biosciences | 137787 | Consumable |
| Chitosan | Sigma-Aldrich | 448869 | Consumable |
| Acetic Acid | Sigma-Aldrich | 320099 | Consumable |
| N-Octanol | Acros Organics | 150630025 | Consumable |
| Sorbitan-Mono-Oleate | Sigma-Aldrich | S6760 | Consumable |
| Potassium Hydroxide | Sigma-Aldrich | P1767 | Consumable |
| Acetone | Fisher Scientific | L-4859 | Consumable |
| Ethanol | Sigma-Aldrich | 270741 | Consumable |
| Trinitro Benzenesulfonic Acid | Sigma-Aldrich | P2297 | Consumable |
| Hydrochloric Acid | Sigma-Aldrich | 320331 | Consumable |
| Ethyl Ether | Sigma-Aldrich | 472-484 | Consumable |
| 8-μm Tissue Culture Plate Inserts | BD Biosciences | 353097 | Consumable |
| 1.5-ml Microcentrifuge Tubes | Fisher Scientific | 05-408-129 | Consumable |
| MTT Reagent | Invitrogen | M6494 | Consumable |
| Dimethyl Sulfoxide | Sigma-Aldrich | D8779 | Consumable |
| Qtracker Cell Labeling Kit(Q Tracker 655) | Molecular Probes, Life Technologies | Q2502PMP | Consumable |
| Type 1 Collagen | Travigen | 3447-020-01 | Consumable |
| Sodium Hydroxide | Sigma-Aldrich | S8045 | Consumable |
| 12-Well Tissue Culture Plates | BD Biosciences | 353043 | Consumable |
| Fibrinogen | Sigma-Aldrich | F3879 | Consumable |
| Thrombin | Sigma-Aldrich | T6884 | Consumable |
| Benztriazole Derivative of Polyethylene | Sunbio | DE-034GS | Consumable |
| Tris Buffer Tablet (pH 7.6) | Sigma-Aldrich | T5030 | Consumable |
| Centrifuge | Eppendorf | 5417R | Equipment |
| Orbital Shaker | New Brunswick Scienctific | C24 | Equipment |
| Humidified Incubator with Air-5% CO2 | Thermo Fisher Scientific, Inc. | Model 370 | Equipment |
| Overhead Stirrer | IKA | Visc6000 | Equipment |
| Magnetic Stirrer | Corning | PC-210 | Equipment |
| Vacuum Desiccator | - | - | Equipment |
| Particle Size Analyzer | Malvern Instruments | STP2000 Spraytec | Equipment |
| Water Bath | Fisher Scientific | Isotemp210 | Equipment |
| Spectrophotometer | Beckman Coulter Inc. | Beckman Coulter DU 800UV/Visible Spectrophotometer | Equipment |
| Vortex | Diagger | 3030a | Equipment |
| Microplate Reader | Molecular Devices | SpectraMax M2 | Equipment |
| Light/Fluorescence Microscope | Olympus Corporation | IX71 | Equipment |
| Confocal Microscope | Olympus Corporation | FV-500 Laser Scanning Confocal Microscope | Equipment |
| Scanning Electron Microscope | Carl Zeiss, Inc. | Leo 435 VP | Equipment |
| Transmission Electron Microscope | JEOL | JEOL 1230 | Equipment |
Odniesienia
- Natesan, S. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng. Part A. 17, 941-953 (2011).
- Nuttelman, C. R., Tripodi, M. C., Anseth, K. S. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 24, 208-218 (2005).
- Benoit, D. S. Integrin-linked kinase production prevents anoikis in human mesenchymal stem cells. J Biomed Mater Res A. 81, 259-268 (2007).
- Willerth, S. M., Sakiyama-Elbert, S. E. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. , (2008).
- Natesan, S. Adipose-derived stem cell delivery into collagen gels using chitosan microspheres. Tissue Eng. Part A. 16, 1369-1384 (2010).
- Bubnis, W. A., Ofner, M. C. The determination of epsilon-amino groups in soluble and poorly soluble proteinaceous materials by a spectrophotometric method using trinitrobenzenesulfonic acid. Anal. Biochem. 207, 129-133 (1992).
- Zhang, G. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 12, 9-19 (2006).
- Bornstein, M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 7, 134-137 (1958).
- Zhang, G. Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta Biomater. 6, 3395-3403 (2010).
- Rochon, M. H. Normal human epithelial cells regulate the size and morphology of tissue-engineered capillaries. Tissue Eng. Part A. 16, 1457-1468 (2010).
- Liu, H., Collins, S. F., Suggs, L. J. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 27, 6004-6014 (2006).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone