Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
An Effective Manual Deboning Method To Prepare Intact Mouse Nasal Tissue With Preserved Anatomical Organization
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Streszczenie
The mammalian nose is a multi-functional organ with intricate internal structures. The nasal cavity is lined with various epithelia such as olfactory, respiratory, and squamous epithelia which differ markedly in anatomical locations, morphology, and functions. In adult mice, the nose is covered with various skull bones, limiting experimental access to internal structures, especially those in the posterior such as the main olfactory epithelium (MOE). Here we describe an effective method for obtaining almost the entire and intact nasal tissues with preserved anatomical organization. Using surgical tools under a dissecting microscope, we sequentially remove the skull bones surrounding the nasal tissue. This procedure can be performed on both paraformaldehyde-fixed and freshly dissected, skinned mouse heads. The entire deboning procedure takes about 20-30 min, which is significantly shorter than the experimental time required for conventional chemical-based decalcification. In addition, we present an easy method to remove air bubbles trapped between turbinates, which is critical for obtaining intact thin horizontal or coronal or sagittal sections from the nasal tissue preparation. Nasal tissue prepared using our method can be used for whole mount observation of the entire epithelia, as well as morphological, immunocytochemical, RNA in situ hybridization, and physiological studies, especially in studies where region-specific examination and comparison are of interest.
Wprowadzenie
The mammalian nasal cavity contains various types of tissues and organs that serve distinct functions. The nasal cavity makes up the entry portion of the upper respiratory tract, which allows air travel into and out of the lungs. Inhaled air passes through the nasal cavity where it undergoes temperature and humidity conditioning 1 as well as cleaning or filtering to remove irritating and toxic substances and infectious microorganisms 2. Both treatments are carried out by nasal epithelia and subepithelial tissues, including glands and vessels and are critical for protecting the lower airways and the lungs. In addition to its role in respiration and epithelial defense, the nasal tissue also contains peripheral sensory apparatuses of the olfactory and trigeminal systems, which detect a wide range of chemical substances in the passing air. Depending on which system is activated, sensory detection of chemicals in the nose can elicit either a sense of smell, irritation, or pain 3,4.
The peripheral olfactory system is complex and made up of several anatomically separated olfactory sensory organs within the nasal cavity. Among them, the main olfactory epithelium (MOE) is the largest, which makes up approximately 45-52% of the nasal epithelia in rodents 5 and is located in the posterior region. In the anteroventral region, there is a pair of tubular structures known as the vomeronasal organ 6, which sit along each side of the nasal septum. Two additional small groupings of olfactory sensory neurons, known as the septal organ of Masera 7,8 and the Gruneberg ganglion 9, reside along the ventral septum and the dorsal entry region of the nasal cavity, respectively. These peripheral organs contain neuro-epithelia with distinctive features in morphology, cell marker expression, and physiological function. Together they detect thousands of odor molecules with exquisite sensitivity 10-12.
In addition to the olfactory sensory organs, the nasal cavity also houses other sensory systems. It is known that peptidergic trigeminal nerve fibers are present in the nasal epithelium, especially the respiratory epithelium 13,14. Some of these fibers detect irritating and toxic chemicals and are responsible for initiating protective reflexes such as coughing and sneezing 4,15. Irritating odorous and bitter compounds can also be detected by a recently discovered population of solitary chemosensory cells (SCCs), many of which are innervated by trigeminal nerve fibers 16-19. These SCCs are located in higher density in the entry region of the nasal cavity and vomeronasal entry ducts, hinting that they may also serve a protective function 16-18. Thus, nasal epithelia can differ substantially in function, morphology, and cell composition depending on their anatomical locations.
Even within a single and specialized epithelium, there are regional differences. The MOE is one such example. The MOE lines various turbinates, which are complicated and curled structures. Because of them, different regions of the MOE experience different air flow rates, and thus, different diffusion and clearance rates of airborne odor molecules 20. Also, it is known that olfactory sensory neurons (OSNs) expressing a given odor receptor are located in one of four circumvented zones of the MOE 21,22. How this location difference affects an OSN’s response to odorants is largely not known. In addition, some OSN populations exhibit regional preference. Guanylyl cyclase-D (GC-D)-expressing OSNs have zonal distributions favoring the cul-de-sac regions of the ectoturbinates 23,24. More recently, we found a subpopulation of canonical OSNs that expresses transient receptor potential channel M5 (trpM5) and is preferentially located in the lateral and ventral regions 25. These results indicate that MOE is not uniform. However, how these regional differences affect olfactory coding is not understood. This is in part because thorough physiological investigation of the MOE and the nose has been limited by the difficulty of obtaining intact nasal epithelia with preserved anatomical organization using current methods.
The nasal epithelia are predominantly surrounded by the anterior bones of the skull, including the nasal, maxilla, palatine, zygomatic, and ethmoid bones. In adult mice and other rodent models, these bones are hard and difficult to remove without damaging the closely associated nasal tissue, particularly the delicate turbinates. Often, chemical-based decalcification is used to soften bones to allow cryosectioning of nasal tissues for morphological, immunohistochemical, and in situ hybridization studies; however, depending on the age of the animal, the decalcification process can last overnight up to 7 days 24,26-28. This treatment is also limited because it requires tissue be fixative-preserved. Additionally, chemical decalcification can be harsh and affect the immunolabeling of some sensitive antibodies 29,30. For physiological studies, live tissue is required, and thus, these experiments are often conducted on isolated OSNs or MOE slices obtained from neonates whose skull bones are thin and soft 17,31,32. Physiological studies can also utilize whole mount preparations by splitting the head 25,33,34, but usually only the medial surface of the nose is easily accessible, limiting physiological recordings on other areas.
Here, we describe an effective, manual deboning method to prepare intact nasal tissues with preserved original anatomical organization and morphology. We sequentially remove the major bones of the anterior skull under a dissection microscope to expose an almost entirely intact nasal epithelium while keeping the thin turbinate bones intact unless the mice are very old and cryosectioning is needed. We also extend the method to preserve the connection between the nasal tissues and olfactory bulbs, as well as the rest of the brain, thus facilitating simultaneous examination of both peripheral and central circuits. Our method can be used to prepare paraformaldehyde-fixed, as well as fresh, live nasal tissue. Thus, our method is expected to facilitate morphological, immunohistochemical and physiological studies of respiration, olfaction, and nasal damage and illness.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Mouse Nose Preparation
We used adult C57BL/6 background mice in this study. All animal care and procedures are approved by the Animal Care and Use Committees (IACUC) of University of Maryland, Baltimore County.
1.1 Acquiring the nose from paraformaldahyde-fixed mice
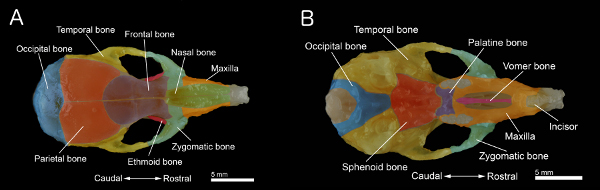
Figure 1. Bones of a mouse skull. A: Dorsal view of the skull. B: Ventral view of the skull with the mandible removed. The skull was prepared from a 40-day old mouse. Individual bones are colored for better visualization. Click here to view larger figure.
- Perfuse transcardially to fix individual mice following the protocol of Lin, et al., (2008)16. Briefly, mice were deeply anesthetized with tribromoethanol (Avertin 250 μg/g body weight), perfused transcardially with 0.1M phosphate buffer (PB, 30-50 ml), followed by a phosphate buffered fixative containing 3% paraformaldehyde, 19 mM L-lysine monohydrochloride, and 0.23% sodium m-periodate (approximately 35-50 ml). One can also follow the steps in the JoVE article for animal perfusion 35.
- Use a pair of scissors to cut off mandible (or the lower jaw) and remove the skin on the head.
- Separate the entire head from the rest of the body.
- Remove the palate. Also, clean and remove the remaining connective tissue and muscle on the surface of the skull to obtain the specimen shown in Figures 1A and 1B.
- Under a dissection microscope, remove the skull bone covering the brain and olfactory bulbs. Trim off excess tissue and bones. Note, for the extended tissue preparation in which the brain and nose remain connected, only the skull bones are removed. For immunohistochemical experiments, the tissue was post-fixed for 1.5 hr and transferred to 0.1M phosphate buffered saline (PBS) with 25% sucrose overnight. Nasal tissue should be kept humidified throughout the dissection by dipping it in the buffered sucrose solution several times.
1.2 Acquiring the nose from freshly euthanized mice
- Individual mice were transferred to a clean cage and exposed to CO2 gas, which was followed by cervical dislocation 5 min after the final breath. To reduce the blood in the nose tissue, use a pair of scissors to open the chest and cut the heart to allow blood to drain.
- Repeat steps 1.1.2 to 1.1.5, except the post-fixation and cryoprotection with 25% sucrose. The specimen should be kept humidified and maintained with Tyrode’s saline containing (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 Na pyruvate, 10 D-glucose, and 10 N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid buffer (HEPES, adjusted at pH 7.4). Alternatively, fold a piece of Kimwipes, soak it with Tyrode’s solution, and place beneath the specimen while dissecting and occasionally dip the specimen in the Tyrode's solution or drop some solution onto the tissue to keep it humidified to maintain the viability of the cells and tissues.
2. Incisor, Anterior Vomer and Maxilla Bone Removal
- Start from a ventral viewpoint. Locate the vomer bone and break the vomer bone along its length using a rongeur or forceps with teeth to break the ventral most part of the bone.
- Using serrated forceps, remove the broken segments of the vomer bone by gently plying the bone fragments away from the vomeronasal organ (VNO). Note, for mice less than a month old, or if only interested in the MOE, these two steps can be skipped.
- Hold the head firmly with forceps. Use the rongeur to break the front portion of both incisors and the jointed region of anterior maxilla between the two incisors and the vomer bone.
- Break the ventral portion of the right maxilla at the region just anterior to the zygomatic arch up to the level of the dorsal zygomatic plate.
- Flip over the nose for a dorsal viewpoint. Use the rongeur to break the right maxilla anterior of the dorsal zygomatic plate. The entire right maxilla anterior of the zygomatic plate should be loose. Use the fine forceps to gently separate any nasal tissue underlying the maxilla, and then gently lift away the bone fragment.
- Repeat steps 2.4 and 2.5 to loosen the left incisor and left anterior maxilla and remove them after the nasal bone has been removed.
3. Nasal Bone and Dorsal Zygomatic Plate Removal
- Use the serrated forceps or the rongeur to remove the remainder of the frontal bones just caudal to the nasal bones. After removing this piece of bone, the caudal part of the nasal bone can be gripped using forceps.
- Use the rongeur to break the anterior portion of the zygomatic arch, which is connected to the zygomatic plate.
- Take the fine forceps at the lateral edge of the dorsal end of the zygomatic plate and gently flip the bone and remove it. If the bone is not loose, use the rongeur to clamp it gently to loosen it for removal.
- Use the fine forceps or a razor blade to loosen the medial suture between the right and left nasal bones.
- Use the serrated forceps to grip the caudal end of the right nasal bone. Gently move the bone from side to side to separate it from underlying tissue. It is useful to move the forceps along the caudal third of the nasal bone for moving the bone side to side. As the nasal bone separates, slowly lift the bone from the caudal end.
- While the nasal bone is slightly lifted, tilt the bone laterally to reveal a lateral outcropping of the bone that is lined with thin respiratory epithelial tissue. Use fine forceps to gently release this tissue from the nasal bone. Continue to lift the nasal bone. When the bone is completely separated from underlying tissue, use scissors to cut off the nasal bone at the rostral end.
- Repeat steps 3.1, 3.5 and 3.6 for the removal of left nasal bone.
- Repeat steps 3.2 and 3.3 for the left side of the nose.
4. Lateral Zygomatic Plate Removal
- Remove the zygomatic plate one side at a time. Either zygomatic plate can be removed first.
- From a ventral viewpoint, break the maxilla inferior to the zygomatic arch until break reaches the zygomatic arch.
- From a dorsal viewpoint, gently grab the zygomatic arch and lift forward and lateral. If the zygomatic plate is still attached to any tissue, take the fine forceps and gently sever the connections between the tissue and the bone.
- Repeat for the zygomatic plate on the other side of the nose.
5. Orbit Bone Removal
- From a ventral viewpoint, break the palantine bone between the molars of the nose.
- Use the rongeur to break the 3 molars and the maxilla on each side of the nose.
- Break and remove any remaining thick pieces of bone ventral and posterior to the turbinates on each side of the nose.
6. Ethmoid Bone Removal
- Break any portion of the ethmoid bone protruding caudal to the turbinates. This is necessary to avoid the loss of turbinate tissue when removing thin pieces of the ethmoid bone covering the turbinates.
- For the right side of the nose, place the fine forceps at the anterior edge of the ethmoid bone and gently remove it. If a portion of the bone remains, repeat this procedure until all the thin bone covering the turbinates has been removed. The turbinate bones do not need to be removed in most of the preparations. In aged mice, the cribriform plate becomes brittle. If cryosectioning of the nasal tissue is needed, remove small pieces of the plate with fine forceps to reduce the potential damage caused by the bone.
- Repeat step 6.2) for the left side of the nose.
- Remove any remaining bone fragments prior to sectioning. Note: in animals more than a year-old, the posterodorsal region of the septum bone is somewhat thick and hard. One can remove this portion using fine forceps. Insert the tip of the forceps into both side of the bone to separate the dorsal portion of the septum bone and the lining epithelial tissue. Use one pair of forceps to grab the bone and hold the specimen. Use another pair of forceps to break the upper bony portion from the lower cartilaginous portion of the septum and gently remove it.
7. Nose Preparation for Cryosectioning
- Set up the aspirator vacuum pump.
- Place the nose in an embedding mold. Submerge the nose in OCT media.
- Use a vacuum to remove air bubbles trapped within the nose tissue. This process takes up to 5 min.
- After removing air bubbles, set the tissue in the desired orientation.
- Freeze the OCT and tissue in the mold using dry ice. The embedded tissue can then be cryosectioned immediately or stored at -80 °C for future use.
Access restricted. Please log in or start a trial to view this content.
Wyniki
Using this method, we can reliably obtain almost entirely intact nasal tissue. Figure 2A shows an image of adult nasal specimen from a paraformaldehyde-fixed head. In this specimen, all four sub-olfactory sensory organs, including the MOE, septal organ, the Gruneberg ganglion, and VNO, are intact. Also, the respiratory epithelia and subepithelial tissues, such as glands and vessels, are preserved. We have successfully used this method in a number of studies in which we investigated morphology, distri...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Here, we demonstrated a step-by-step procedure for isolating intact olfactory and respiratory tissue from the mouse nose by sequentially removing the surrounding bones while sparing the tissue below. We show that careful bone removal can preserve even the most delicate tissues in their entirety. We also share insight into possible modifications of this technique, in which we isolate both the brain and nose tissue together to preserve the nerve connection. This new method provides a means for isolating whole olfactory ...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors declare that they have no competing financial interests.
Podziękowania
This work was supported by research grants (NIH/NIDCD 009269, 012831 and ARRA administrative supplement NIH grants) to Weihong Lin. We especially thank Mr. Tim Ford at UMBC for his technical assistance in videotaping and processing. We also wish to thank Dr. Daphne Blumberg, Ms. Chere Petty at UMBC and Mr. Nicholas McCollum from Olympus America Inc. for their equipment assistance in videotaping.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Dissection | |||
| Rongeur, 1.0 mm Jaw width | World Precision Instruments (WPI) | 501333 | |
| Fine forceps, Dumont 3 | WPI | 503235 | |
| Fine forceps, Dumont 55 | WPI | 14099 | |
| Fine forceps, Dumont AA | Fine Science Tools (FST) | 11210-20 | |
| Specimen forceps, Serrated | VWR | 82027-440 | |
| Operating scissors | WPI | 501753 | |
| Iris scissors, Straight | Miltex | V95-304 | |
| Dissection microscope | Olympus | SZ40 | |
| [header] | |||
| Tissue embedding | |||
| Optimum cutting temperature (OCT) compound | Sakura Finetek | 4583 | |
| Plastic embedding mold | VWR | 15160-215 | |
| Aspirator vacuum pump | Fisher Scientific | 09-960-2 | |
| [header] | |||
| Section staining | |||
| Neutral red | ACROS Organic | CAS 553-24-2 | Nuclei staining |
Odniesienia
- Naclerio, R. M., Pinto, J., Assanasen, P., Baroody, F. M. Observations on the ability of the nose to warm and humidify inspired air. Rhinology. 45, 102-111 (2007).
- Bjermer, L. The nose as an air conditioner for the lower airways. Allergy. 54, Suppl 57. 26-30 (1999).
- Firestein, S. How the olfactory system makes sense of scents. Nature. 413, 211-218 (2001).
- Bryant, B., Silver, W. L. Chemisthesis: The common chemical sense. , 2nd, Wiley-Liss. (2000).
- Gross, E. A., Swenberg, J. A., Fields, S., Popp, J. A. Comparative morphometry of the nasal cavity in rats and mice. J. Anat. 135, 83-88 (1982).
- Halpern, M. The organization and function of the vomeronasal system. Annu. Rev. Neurosci. 10, 325-362 (1987).
- Rodolfo-Masera, T. Su l'esquoestizenza di un particulare organo olfacttivo nel setto nasale della cavia e di altri roditori. Arch. Ital. Anat. Embryol. 48, 157-212 (1943).
- Levai, O., Strotmann, J. Projection pattern of nerve fibers from the septal organ: DiI-tracing studies with transgenic OMP mice. Histochemistry and Cell biology. 120, 483-492 (2003).
- Storan, M. J., Key, B. Septal organ of Gruneberg is part of the olfactory system. J. Comp. Neurol. 494, 834-844 (2006).
- Restrepo, D., Arellano, J., Oliva, A. M., Schaefer, M. L., Lin, W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm. Behav. 46, 247-256 (2004).
- Breer, H., Fleischer, J., Strotmann, J. The sense of smell: multiple olfactory subsystems. Cell Mol. Life Sci. 63, 1465-1475 (2006).
- Munger, S. D., Leinders-Zufall, T., Zufall, F. Subsystem organization of the mammalian sense of smell. Annu. Rev. Physiol. 71, 115-140 (2009).
- Finger, T. E., St Jeor, V. L., Kinnamon, J. C., Silver, W. L. Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J. Comp. Neurol. 294, 293-305 (1990).
- Papka, R. E., Matulionis, D. H. Association of substance-P-immunoreactive nerves with the murine olfactory mucosa. Cell Tissue Res. 230, 517-525 (1983).
- Baraniuk, J. N., Kim, D. Nasonasal reflexes, the nasal cycle, and sneeze. Curr. Allergy Asthma Rep. 7, 105-111 (2007).
- Lin, W., Ogura, T., Margolskee, R. F., Finger, T. E., Restrepo, D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J. Neurophysiol. 99, 1451-1460 (2008).
- Ogura, T., et al. Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J. Neurophysiol. 106, 1274-1287 (2011).
- Finger, T. E., et al. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proceedings of the National Academy of Sciences of the United States of America. 100, 8981-8986 (2003).
- Gulbransen, B. D., Clapp, T. R., Finger, T. E., Kinnamon, S. C. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J. Neurophysiol. 99, 2929-2937 (2008).
- Zhao, K., Dalton, P., Yang, G. C., Scherer, P. W. Numerical modeling of turbulent and laminar airflow and odorant transport during sniffing in the human and rat nose. Chemical Senses. 31, 107-118 (2006).
- Ressler, K. J., Sullivan, S. L., Buck, L. B. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 73, 597-609 (1993).
- Vassar, R., Ngai, J., Axel, R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 74, 309-318 (1993).
- Fulle, H. J., et al. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 92, 3571-3575 (1995).
- Juilfs, D. M., et al. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proceedings of the National Academy of Sciences of the United States of America. 94, 3388-3395 (1997).
- Lin, W., Arellano, J., Slotnick, B., Restrepo, D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. The Journal of Neuroscience: The Official journal of the Society for Neuroscience. 24, 3703-3710 (2004).
- Ishii, T., Omura, M., Mombaerts, P. Protocols for two- and three-color fluorescent RNA in situ hybridization of the main and accessory olfactory epithelia in mouse. J. Neurocyt. 33, 657-669 (2004).
- Lee, A. C., Tian, H., Grosmaitre, X., Ma, M. Expression patterns of odorant receptors and response properties of olfactory sensory neurons in aged mice. Chemical Senses. 34, 695-703 (2009).
- Packard, A., Schnittke, N., Romano, R. A., Sinha, S., Schwob, J. E. DeltaNp63 regulates stem cell dynamics in the mammalian olfactory epithelium. The Journal of Neuroscience: the official journal of the Society for Neuroscience. 31, 8748-8759 (2011).
- Matthews, J. B., Mason, G. I. Influence of decalcifying agents on immunoreactivity of formalin-fixed, paraffin-embedded tissue. Histochem J. 16, 771-787 (1984).
- Athanasou, N. A., Quinn, J., Heryet, A., Woods, C. G., McGee, J. O. Effect of decalcification agents on immunoreactivity of cellular antigens. J. Clin. Pathol. 40, 874-878 (1987).
- Hegg, C. C., Irwin, M., Lucero, M. T. Calcium store-mediated signaling in sustentacular cells of the mouse olfactory epithelium. Glia. 57, 634-644 (2009).
- Spehr, M., et al. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. The Journal of Neuroscience: the official journal of the Society for Neuroscience. 26, 1961-1970 (2006).
- Ma, M., Chen, W. R., Shepherd, G. M. Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. J. Neurosci. Methods. 92, 31-40 (1999).
- Cygnar, K. D., Stephan, A. B., Zhao, H. Analyzing responses of mouse olfactory sensory neurons using the air-phase electroolfactogram recording. J. Vis. Exp. (37), e1850(2010).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. J. Vis. Exp. (65), e3564(2012).
- Lin, W., Margolskee, R., Donnert, G., Hell, S. W., Restrepo, D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proceedings of the National Academy of Sciences of the United States of America. 104, 2471-2476 (2007).
- Lin, W., Ezekwe, E. A., Zhao, Z., Liman, E. R., Restrepo, D. TRPM5-expressing microvillous cells in the main olfactory epithelium. BMC Neurosci. 9, 114(2008).
- Ogura, T., Krosnowski, K., Zhang, L., Bekkerman, M., Lin, W. Chemoreception regulates chemical access to mouse vomeronasal organ: role of solitary chemosensory cells. PLoS One. 5, e11924(2010).
- Sathyanesan, A., Feijoo, A. A., Mehta, S. T., Nimarko, A. F., Lin, W. Expression profile of G-protein βγ subunit gene transcripts in the mouse olfactory sensory epithelia. Frontiers in Cellular Neuroscience. 7, 84(2013).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone