Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Studying DNA Looping by Single-Molecule FRET
W tym Artykule
Podsumowanie
This study presents a detailed experimental procedure to measure looping dynamics of double-stranded DNA using single-molecule Fluorescence Resonance Energy Transfer (FRET). The protocol also describes how to extract the looping probability density called the J factor.
Streszczenie
Bending of double-stranded DNA (dsDNA) is associated with many important biological processes such as DNA-protein recognition and DNA packaging into nucleosomes. Thermodynamics of dsDNA bending has been studied by a method called cyclization which relies on DNA ligase to covalently join short sticky ends of a dsDNA. However, ligation efficiency can be affected by many factors that are not related to dsDNA looping such as the DNA structure surrounding the joined sticky ends, and ligase can also affect the apparent looping rate through mechanisms such as nonspecific binding. Here, we show how to measure dsDNA looping kinetics without ligase by detecting transient DNA loop formation by FRET (Fluorescence Resonance Energy Transfer). dsDNA molecules are constructed using a simple PCR-based protocol with a FRET pair and a biotin linker. The looping probability density known as the J factor is extracted from the looping rate and the annealing rate between two disconnected sticky ends. By testing two dsDNAs with different intrinsic curvatures, we show that the J factor is sensitive to the intrinsic shape of the dsDNA.
Wprowadzenie
Understanding the mechanical properties of dsDNA is of fundamental importance in basic sciences and engineering applications. The structure of dsDNA is more complicated than a straight helical ladder because roll, tilt, and twist angles between successive base pairs can vary with sequence. Thermal fluctuations can cause dsDNA to undergo diverse modes of conformational fluctuations such as bending, twisting and stretching. Transitions such as melting and kinking can also occur in extreme conditions.
Among these motions, dsDNA bending has the most noticeable biological impact1. dsDNA bending is associated with gene repression or activation by bringing two distant sites close to each other. It also plays an important role in DNA packaging inside the cell nucleus or a viral capsid. Bending deformation of dsDNA can be visualized experimentally by high-resolution microscopy (AFM2 and TEM3), and the thermodynamics and kinetics can be studied by looping assays, which chemically link juxtaposed sites of the dsDNA.
One such assay is ligase-dependent cyclization4. In this assay, dsDNA molecules with ‘sticky’ (cohesive) ends are circularized or dimerized by DNA ligase. By comparing the rates of circle and dimer formation, one can obtain an effective molar concentration of one end of the DNA in the vicinity of the other end, which is known as the J factor. This J factor is dimensionally equivalent to the probability density of finding one end of the DNA at a short distance from the other end, and thus reflects the flexibility of the DNA. Measuring the J factor as a function of DNA length reveals many characteristics about DNA mechanics including the persistence length4,5.
The worm-like chain (WLC) model has been widely regarded as the canonical polymer model for dsDNA mechanics based on its success in explaining the force-extension curves obtained in DNA pulling experiments6, and correctly predicting the J factors of dsDNAs longer than 200 bp7. However, using the cyclization assay on dsDNA molecules as short as 100 bp, Cloutier and Widom measured the J factors to be several orders of magnitude higher than the WLC model prediction8. A year later, Du et al. produced J factors in agreement with the WLC model using the cyclization assay with lower concentrations of ligase and attributed the anomalous result from the Widom group to high ligase concentrations used9. This controversy exemplifies the unavoidable influence of DNA ligase on cyclization kinetics when using the conventional assay9. Moreover, DNA ligase can also affect DNA structure and stiffness through nonspecific binding10,11.
To eliminate the technical concerns of protein-dependent looping assays, we recently demonstrated a protein-free looping assay based on Fluorescence Resonance Energy Transfer (FRET)12. In this method, looped conformations are detected by FRET between the donor and acceptor attached near the sticky ends of a DNA molecule. An objective-type total internal reflection fluorescence microscope (TIRFM) is used to record trajectories of reversible looping and unlooping events from surface-immobilized single DNA molecules for a prolonged period of time. This method features PCR-based assembly of DNA molecules to generate mismatch-free DNA molecules, which is a crucial improvement over a similar method by Vafabakhsh and Ha13. The single-molecule aspect of this protocol allows measurement of distributions in addition to ensemble averages while the FRET aspect allows one to measure DNA looping dynamics repeatedly from the same molecule, even in conditions that can impair ligase activity.
The TIRFM setup is shown in Figure 1. A custom-designed specimen stage is placed over an Olympus IX61 microscope body. 532 nm and 640 nm lasers are introduced from the side and are reflected by tiny elliptical mirrors14 into the high NA objective to achieve critical angle of incidence at the coverslip-water interface. We note that more widespread through-objective TIR using dichroic mirrors or prism-based TIR setups can also be used for this FRET application. The fluorescence image formed by the microscope is split into donor and acceptor images by a dichroic mirror. They are then re-imaged onto two halves of an EMCCD. Additional long-pass emission filters are used to reduce background signal.
Temperature control is essential for acquiring reproducible kinetic data. For temperature control, the objective is separated from the nosepiece of the microscope body to minimize heat transfer, and water from a temperature controlled chiller/heater is circulated through a brass collar that tightly fits around the interior metal beneath the objective jacket. This setup is able to achieve robust temperature control at the coverslip surface between 15 and 50 °C (Figure 2). In this work, the sample temperature was maintained at 24 °C.
The following protocol presents the step-by-step procedure for DNA construction, estimation of DNA shape, single-molecule experiment, and J factor determination.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. dsDNA Sample Preparation
- Design globally curved DNAs by repeating a 10-mer sequence. For example, 5’-GTGCCAGCAACAGATAGC - (TTTATCATCCTTTATCATCCX)7 - TTTCATTCGAGCTCGTTGTTG-3’ is a 186-bp curved DNA where X is a random extra base and the sequence flanking the repeating 10-mer sequence are adapter sequences.
NOTE: In this example two 10-mers with opposite preferences to nucleosome formation based on a large-scale nucleosome occupancy study by Kaplan et al.15 were chosen. Since the helical repeat of dsDNA is close to 10 bp, any net deflection of the helical axis of the 10-mer will accumulate to produce a shape like a circular arc (Figure 3A). Since the helical period is closer to 10.5 bp, an extra base is inserted after every two repeats to keep the curved structure as planar as possible. These sequences shorter than 200 bp can be ordered from companies that offer gene synthesis service. It is convenient to flank these sequences with common adapter sequences for subsequent steps. The procedure is schematized in Figure 3B. - Perform PCR with Primer 1 (GTGCCAGCAACAGATAGC) and Primer 2 (/5Cy3/TAAATTCCTACAACAACGAGCTCGAATG). NOTE: Primer 2 is labeled with the FRET donor Cy3 at the 5' end. A typical PCR recipe and cycling protocol are presented in Tables 1 and 2.
- Perform PCR with Primer 3 (/5BioTEG/GAAACATAG/iCy5/GAATTTACCGTGCCAGCAACAGATAGC) and Primer 4 (CAACAACGAGCTCGAATG). NOTE: Primer 3 is labeled with the FRET acceptor Cy5 through the backbone and the biotin-linker at the 5' end. PCR recipe and cycling protocol are as above.
- Purify the PCR products using a PCR cleanup kit.
- Mix the Cy3-labeled product and the Cy5-labeled product in a buffer for strand exchange (100 mM NaCl, 10 mM Tris-HCl pH 7.0, 1 mM EDTA) at final concentrations of 0.4 µM and 0.1 µM, respectively. NOTE: The excess Cy3-DNA increases the concentration of the duplex carrying both Cy3 and Cy5 as a result of strand exchange.
- Exchange strands by incubating at 98.5 °C for 2 min, gradually cooling to 5 °C with ramp rate of 0.1 °C/sec, and incubating at 5 °C for 2 hr.
2. Gel Electrophoresis to Detect dsDNA Curvature
- Pour the polyacrylamide gel16,17 by mixing acrylamide and bis-acrylamide solutions at 29.2:0.8 ratio, 5% (w/v) in 1X Tris/Borate/EDTA (TBE) buffer at pH 8.0. Note: 10 ml of gel solution contains: 1.217 ml 40% acrylamide, 0.667 ml 2% bis-acrylamide, 1 ml TBE 10X, 100 L ammonium persulfate (APS) 10%, 10 L TEMED, and the rest is dH2O. Full solidification of the gel takes about 30 min.
- Load the polyacrylamide gel with DNA samples and the DNA ladder in 1X loading buffer (5% glycerol, 0.03% (w/v) bromophenol blue) and run the gel at 5-8 V/cm at 4 °C for 45 min or until the dye front approaches the end of the gel.
- Stain the gel using 1X TBE buffer that contains 0.5 g/ml ethidium bromide for 30 min. Identify the DNA bands under UV illumination. Compare band positions with the size marker (100 bp DNA ladder) to calculate the apparent sizes of the DNA molecules. NOTE: Curved DNAs generally move slower than straight DNAs.
3. Flow Cell Preparation
- Drill 6-7 pairs of holes along two opposite edges of a glass slide (3’’ x 1’’) using a drill press and diamond drill bits. After drilling, rub the slide in flowing water to remove visible glass powder. NOTE: The holes serve as perfusion inlets and outlets. During drilling, cooling the slide with water is important to prevent cracking.
- Place the slides upright in a glass jar and fill it with water. Sonicate for 15 min and transfer them into another glass jar dedicated for acetone cleaning. Fill it with acetone and sonicate for 15 min. Rinse the slides with ethanol using a spray bottle and then with water. Place them in a polypropylene jar, fill it with 5 M potassium hydroxide, and sonicate for 15 min. Finally, sonicate the slides in water for 15 min. Clean the coverslips (no. 1, 24 x 40 mm) using the same protocol. NOTE: Cleaned slides and coverslips can be stored in dH2O for long term use.
- Mix 1 mg of biotin-PEG-silane (MW 3400) with 80 mg of mPEG-silane (MW 2000) in 340 L of 0.1 M sodium bicarbonate solution. Mix well and centrifuge the mixture briefly to get rid of bubbles. NOTE: The functionalization of the surface with polyethylene glycol (PEG) helps reduce nonspecific binding of DNA to the surface.
- Put 80 L of the PEG solution on each slide and gently lower a coverslip over it. Wait for 45 min. Separate the coverslip from the slide with tweezers, rinse them with copious amount of dH2O and let them dry in open air.
- Place thin strips of double-stick tape across the slide to form channels. Align a coverslip over it and firmly press the coverslip against the slide to form liquid-tight channels. Use 5 minute epoxy to seal the edges of the channels.
4. Preparation of Trolox Solution
- Put ~30 mg Trolox and 10 ml dH2O in a flask and use a magnet stir bar to stir the solution in open air for 18 hr.
- Filter the solution using a 0.2 µm filter and adjust the pH to 7 by adding ~6 µl of 1 M Tris base (pH 11). Note: Trolox is an anti-blinking reagent which is commonly used in single-molecule studies18. The antifading action of Trolox comes from its oxidized derivative which is present in partially degraded Trolox solution19. Quickly dissolving Trolox in methanol or high pH Tris solution should be avoided because of inefficient oxidation.
5. Single-molecule Imaging
- Inject 15 µl of NeutrAvidin solution (0.5 mg/ml) into the channel and wait for 2 min before rinsing with 100 µl of T50 buffer (10 mM Tris-HCl, 50 mM NaCl, pH 7.0).
- Inject 50 µl of DNA sample (50-100 pM) into the channel. Wait for 5 min and rinse the unbound DNA away with 100 L of T50 buffer. NOTE: DNA molecules will specifically bind to the surface through the NeutrAvidin-biotin interaction.
- Fill the channel with the imaging buffer that contains an oxygen scavenging system20 (100 mM PCD, 5 mM PCA, 1 mM Trolox, and 500 mM NaCl).
- Set the EMCCD in frame-transfer mode to stream 2 x 2 binned images (256 x 256) to the computer at 25 frames per sec.
- Put immersion oil on the microscope objective, and fix the flow cell on the microscope stage using specimen clips. Coarse-adjust the focus by looking at the laser reflection pattern on the wall. Fine-adjust the focus using the live view of fluorescence images on the monitor.
- Begin data acquisition with the 532 nm laser on. Check the live view and adjust the focus if necessary. Stop data acquisition when most molecules have photobleached.
NOTE: In this facility, a lab-written C program to control the microscope and display live images on the monitor as they are saved to the hard disk is used.
6. Image Processing and Data Analysis
NOTE: A time series of 256 x 256 images are processed by a MATLAB code to generate single-molecule time traces of Cy3 and Cy5 intensities. To pair pixels between the donor channel and the acceptor channel of the split-view image, 6-7 pairs of Cy3 and Cy5 spots, each pair from the same molecule evenly dispersed across the field of view, are manually picked, and an affine transformation is calculated using the coordinates of these spots as anchor points.
- Using a MATLAB script, look through all single-molecule time traces that show multiple transitions between low and high FRET signals. Identify the looped and unlooped states.
NOTE: The FRET signal is defined as the Cy5 intensity ( Ia ) divided by the sum of Cy3 and Cy5 intensities ( Ia + Id ). Looped state has high FRET value while unlooped state has low FRET value. The histogram of FRET signals from a single molecule is bimodally distributed because of reversible looping and unlooping. - Find the threshold that separates the two distributions by determining the intersection between the two fitted Gaussian curves.
- Calculate the FRET efficiency as
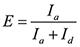 and assign the looped states with high FRET values and the unlooped states with low FRET values.
and assign the looped states with high FRET values and the unlooped states with low FRET values. - Using a MATLAB script, analyze the cumulative number of molecules ( N(t) ) that looped (or reached the high FRET state) at different time lapses since the start of data acquisition. NOTE: Since a dsDNA molecule can start in any conformation of the unlooped state at the start of data acquisition, the rate of increase in the looped population reflects the mean looping rate averaged over initial conformations. Extract the looping rate kloop by fitting N(t) with an exponential function:
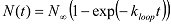 If increases biphasically, it can be fitted with a double exponential function:
If increases biphasically, it can be fitted with a double exponential function: 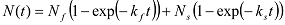 In this case, kloop is obtained from:
In this case, kloop is obtained from: 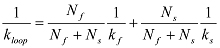 NOTE: Theoretically, the survival probability that a polymer has not looped at time t is not a single or double exponential function12. Exponential fitting is used as a practical means to extract the average looping time.
NOTE: Theoretically, the survival probability that a polymer has not looped at time t is not a single or double exponential function12. Exponential fitting is used as a practical means to extract the average looping time.
7. Determining the J Factor
NOTE: The J factor represents how concentrated one end of a dsDNA is around the other end. It can be determined by interpolating the concentration of one end segment of the DNA that would produce the same reaction rate with the other end segment as the measured looping rate. Experimentally, one end segment is immobilized on the surface, and the other end segment is introduced at a certain concentration c. If the measured annealing rate between the two ends is kanneal, then the J factor21 is given by 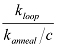 . The annealing rate constant ( kanneal = kanneal / C ) is independent of the probe concentration.
. The annealing rate constant ( kanneal = kanneal / C ) is independent of the probe concentration.
- Flow 20 µl of 30-50 pM biotin-Cy5 oligo (Primer 3) into a NeutrAvidin-coated channel. Rinse the channel with 100 µl T50 to wash away unbound oligos.
- Prepare the imaging buffer as described in part 5 with the addition of Cy3 oligo (Primer 2) at a final concentration of 50 nM. Flow this imaging buffer into the channel.
- While keeping the 532 nm laser on, briefly turn the 640 nm laser on to identify locations of surface-bound Cy5 oligos. Turn off the 640 nm laser and start monitoring the FRET signal.
NOTE: Upon hybridization of a Cy3 oligo to a surface-bound Cy5 oligo, Cy5 fluorescence will arise from FRET. - Using a MATLAB script, analyze the number of molecules which start in the unbound state (low Cy5 intensity) but later turn into the annealed state (high Cy5 intensity) as a function of time from the Cy5 intensity traces.
- Plot this number of annealed molecules vs. time. Fit this curve with a single exponential function (
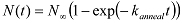 ) to obtain the annealing rate ( kanneal ).
) to obtain the annealing rate ( kanneal ). - Repeat this experiment at different Cy3 oligo concentrations (60, 100, and 180 nM) to confirm linearity between the annealing rate and the reactant concentration. Extract the second-order annealing rate constant ( kanneal ) from the slope.
- Calculate the J factor from
 , where kloop is the looping rate measured in the same buffer condition.
, where kloop is the looping rate measured in the same buffer condition.
Access restricted. Please log in or start a trial to view this content.
Wyniki
DNA molecules used for the looping study consist of a duplex region of variable sequence and length and single-stranded overhangs that are complementary to each other. The overhangs, which are 7-base long, can anneal to each other to capture the looped state. Each overhang contains either Cy3 or Cy5 that is linked in the DNA backbone through amidite chemistry. The Cy5-overhang is also linked with biotin-TEG (15-atom Tetra-Ethylene Glycol spacer) for surface immobilization (see Figure 4A). All these modif...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
A simple single-molecule assay based on FRET was used to study looping kinetics of DNAs of different intrinsic shapes. Curved DNAs can be prepared by repeating a 10-mer sequence in phase with the helical period of 10.5 bp, and their curvatures can be estimated using PAGE. These dsDNAs are designed with sticky ends to allow transient loop stabilization. We extracted the looping rate from the exponential rise in the number of looped molecules over time. The annealing rate constant between the disconnected sticky end segmen...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors declare no conflicts of interest.
Podziękowania
We thank James Waters, Gable Wadsworth and Bo Broadwater for critically reading the manuscript. We also thank four anonymous reviewers for providing useful comments. We acknowledge financial support from Georgia Institute of Technology, the Burroughs Wellcome Fund Career Award at the Scientific Interface, and the student research network grant from NSF Physics of Living Systems.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Small DNA FRAG Extract Kit-100PR | VWR | 97060-558 | |
| Acrylamide 40% solution 500 ml | VWR | 97064-522 | |
| Bis-acrylamide 2% (w/v) solution 500 ml | VWR | 97063-948 | |
| GeneRuler 100 bp DNA Ladder, 100-1,000 bp | Fermentas | SM0241 | |
| Mini Vertical PAGE System | VWR | 89032-300 | |
| Syringe filter 0.2 μm CS50 | VWR | A2666 | |
| Trolox | Sigma-Aldrich | 238813-1G | triplet state quencher |
| Protocatechuic acid (PCA) | Sigma-Aldrich | 08992-50MG | oxygen scavenging system |
| Protocatechuate 3,4-Dioxygenase (PCD) | Sigma-Aldrich | P8279-25UN | oxygen scavenging system |
| mPEG-silane, MW 2,000 1 g | Laysan Bio | MPEG-SIL-2000-1g | |
| Biotin-PEG-Silane, MW 3,400 | Laysan Bio | Biotin-PEG-SIL-3400-1g | |
| Avidin, NeutrAvidin Biotin-binding Protein | Invitrogen | A2666 | |
| Phusion Hot Start High-Fidelity DNA Polymerase | New England Biolabs | F-540L | |
| Gel/PCR DNA Fragments Extraction Kit | IBI Scientific | IB47020 | |
| Premium plain glass microscope slides | Fisher Scientific | 12-544-1 | |
| VWR micro cover glass, rectangular, no. 1 | VWR | 48404-456 | |
| Fisher Scientific Isotemp 1006S Recirculating Chiller/Heater | Fisher Scientific | temperature control | |
| Objective Cooling Collar | Bioptechs | 150303 | temperature control |
| KMI53 Biological Micrometer Measuring Stage | Semprex | KMI53 | |
| High Performance DPSS Laser 532 nm 50 mW | Edmund optics | NT66-968 | Cy3 excitation |
| CUBE Fiber Pigtailed 640 nm, 30 mW, Fiber, FC/APC Connector | Coherent | 1139604 | Cy5 excitation |
| 650 nm BrightLine Dichroic Beamsplitter | Semrock | FF650-Di01-25x36 | splitting dichroic |
| LaserMUX Beam Combiner, reflects 514.5, 532, & 543.5 nm lasers, 25 mm | Semrock | LM01-552-25 | combining dichroic |
| Brightline Fluorescence Filter 593/40 | Semrock | FF01-593/40-25 | Cy3 emission filter |
| 635 nm EdgeBasic LWP longpass Filter, 25 mm | Semrock | BLP01-635R-25 | Cy5 emission filter |
| EMCCD iXon+ | Andor Technology | DU-897E-CS0-#BV | |
| IX51 inverted microscope frame | Olympus | ||
| Objective UApo N 100X/1.49 Oil TIRF | Olympus | ||
| Immersion oil type-F for fluorescence microscopy | Olympus | IMMOIL-F30CC | |
| 2 mm Diameter 45° Rod Lens Aluminum Coated | Edmund optics | 54-092 | miniature mirror |
| 1/4" Travel Single-Axis Translation Stage | Thorlabs | MS-1 | translation of miniature mirror |
| Ø1" Achromatic Doublet, ARC: 400-700 nm, f=200 mm | Thorlabs | AC254-200-A | focusing lens |
| Adjustable Mechanical Slit | Thorlabs | VA100 | |
| Dielectric Mirror | Thorlabs | BB1-E02 | |
| Ø1" Achromatic Doublet, f = 100 mm | Thorlabs | AC254-100-A | relay lens |
| Lens Mount for Ø1" Optics | Thorlabs | LMR1 | |
| Dichroic Filter Mount | Thorlabs | FFM1 | |
| Fixed Cage Cube Platform | Thorlabs | B3C | |
| Kinematic Mount for Ø1" Optics | Thorlabs | KM100 | |
| N-BK7 Plano-Convex Lens, Ø1", f = 40 mm | Thorlabs | LA1422-A | collimating lens |
| N-BK7 Plano-Convex Lense, Ø6.0 mm, f = 15 mm | Thorlabs | LA1222-A | telescope lens |
| N-BK7 Plano-Convex Lense, Ø6.0 mm, f = 150 mm | Thorlabs | LA1433-A | telescope lens |
Odniesienia
- Garcia, H. G., et al. Biological consequences of tightly bent DNA: The other life of a macromolecular celebrity. Biopolymers. 85, 115-130 (2007).
- Wiggins, P. A., et al. High flexibility of DNA on short length scales probed by atomic force microscopy. Nature Nanotechnology. 1, (2006).
- Lionberger, T. A., et al. Cooperative kinking at distant sites in mechanically stressed DNA. Nucleic Acids Research. 41, 6785-6792 (2011).
- Shore, D., et al. DNA flexibility studied by covalent closure of short fragments into circles. Proc Natl Acad Sci U S A. 78, 4833-4837 (1981).
- Geggier, S., Vologodskii, A. Sequence dependence of DNA bending rigidity. Proc Nat Acad Sci U S A. 107, 15421-15426 (1992).
- Smith, S. B., et al. Direct mechanical measurements of the elasticity of single DNA molecules by using magnetic beads. Science. 258, 1122-1126 (1992).
- Peters, J. P., Maher, L. J. DNA curvature and flexibility in vitro and in vivo. Quarterly Reviews of Biophysics. 43, 23-63 (2010).
- Cloutier, T. E., Widom, J. Spontaneous sharp bending of double-stranded DNA. Molecular Cell. 14, 355-362 (2004).
- Du, Q., et al. Cyclization of short DNA fragments and bending fluctuations of the double helix. Proc Natl Acad Sci U S A. 102, 5397-5402 (2005).
- Yuan, C., et al. T4 DNA ligase is more than an effective trap of cyclized dsDNA. Nucl. Acids Res. 35, 5294-5302 (2007).
- Manzo, C., et al. The effect of nonspecific binding of lambda repressor on DNA looping dynamics. Biophysical Journal. 103, 1753-1761 (2012).
- Le, T. T., Kim, H. D. Measuring shape-dependent looping probability of DNA. Biophys. J. 104, 2068-2076 (2013).
- Vafabakhsh, R., Ha, T. Extreme bendability of DNA less than 100 base pairs long revealed by single-molecule cyclization. Science. 337, 1097-1101 (2012).
- Friedman, L., et al. Viewing dynamic assembly of molecular complexes by multi-wavelength single-molecule fluorescence. Biophysical Journal. 91, 1023-1031 (2006).
- Kaplan, N., et al. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature. 458, 362-366 (2009).
- Koo, H. S., Crothers, D. M. Calibration of DNA curvature and a unified description of sequence-directed bending. Proc Nat Acad Sci U S A. 85, 1763-1767 (1988).
- Prosseda, G., et al. A temperature-induced narrow DNA curvature range sustains the maximum activity of a bacterial promoter in vitro. Biochemistry. 49, 2778-2785 (2010).
- Rasnik, I., et al. Nonblinking and long-lasting single-molecule fluorescence imaging. Nature Methods. 3, 891-893 (2006).
- Cordes, T., et al. On the mechanism of Trolox as antiblinking and antibleaching reagent. J. Am. Chem. Soc. 131, 5018-5019 (2009).
- Aitken, C. E., et al. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys J. 94, 1826-1835 (2008).
- Taylor, W. H., Hagerman, P. J. Application of the method of phage T4 DNA ligase-catalyzed ring-closure to the study of DNA structure: II. NaCl-dependence of DNA flexibility and helical repeat. Journal of Molecular Biology. 212, 363-376 (1990).
- Bolshoy, A., et al. Curved DNA without A-A: experimental estimation of all 16 DNA wedge angles. Proc Natl Acad Sci U S A. 88, 2312-2316 (1991).
- Gibson, D. G. Synthesis of DNA fragments in yeast by one-step assembly of overlapping oligonucleotides. Nucl. Acids Res. 37, 6984-6990 (2009).
- Vologodskii, A., et al. Bending of short DNA helices. Artif DNA PNA XNA. 4, (2013).
- Hoover, D. M., Lubkowski, J. DNAWorks: an automated method for designing oligonucleotides for PCR-based gene synthesis. Nucl. Acids Res. 30, (2002).
- Waters, J. T., Kim, H. D. Equilibrium Statistics of a Surface-Pinned Semiflexible Polymer. Macromolecules. 46, 6659-6666 (2013).
- Mills, J. B., et al. Electrophoretic evidence that single-stranded regions of 1 or more nucleotides dramatically increase the flexibility of DNA. Biochemistry. 33, 1797-1803 (1994).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone