Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Quantitative Magnetic Resonance Imaging of Skeletal Muscle Disease
W tym Artykule
Podsumowanie
Neuromuscular diseases often exhibit a temporally varying, spatially heterogeneous, and multi-faceted pathology. The goal of this protocol is to characterize this pathology using non-invasive magnetic resonance imaging methods.
Streszczenie
Quantitative magnetic resonance imaging (qMRI) describes the development and use of MRI to quantify physical, chemical, and/or biological properties of living systems. Neuromuscular diseases often exhibit a temporally varying, spatially heterogeneous, and multi-faceted pathology. The goal of this protocol is to characterize this pathology using qMRI methods. The MRI acquisition protocol begins with localizer images (used to locate the position of the body and tissue of interest within the MRI system), quality control measurements of relevant magnetic field distributions, and structural imaging for general anatomical characterization. The qMRI portion of the protocol includes measurements of the longitudinal and transverse relaxation time constants (T1 and T2, respectively). Also acquired are diffusion-tensor MRI data, in which water diffusivity is measured and used to infer pathological processes such as edema. Quantitative magnetization transfer imaging is used to characterize the relative tissue content of macromolecular and free water protons. Lastly, fat-water MRI methods are used to characterize fibro-adipose tissue replacement of muscle. In addition to describing the data acquisition and analysis procedures, this paper also discusses the potential problems associated with these methods, the analysis and interpretation of the data, MRI safety, and strategies for artifact reduction and protocol optimization.
Wprowadzenie
Quantitative magnetic resonance imaging (qMRI) describes the development and use of MRI to quantify physical, chemical, and/or biological properties of living systems. QMRI requires that one adopt a biophysical model for the system, composed of the tissue of interest and an MRI pulse sequence. The pulse sequence is designed to sensitize the images' signal intensities to the parameter of interest in the model. MRI signal properties (signal magnitude, frequency, and/or phase) are measured and analyzed according to the model. The goal is to produce an unbiased, quantitative estimate of a physical or biological parameter having continuously distributed, physical units of measurement. Often the equations describing the system are analyzed and fitted on a pixel-by-pixel basis, producing an image whose pixel values directly reflect the values of the variable. Such an image is referred to as a parametric map.
A common use of qMRI is the development and application of biomarkers. Biomarkers can be used to investigate a disease mechanism, establish a diagnosis, determine a prognosis, and/or assess a therapeutic response. They may take the form of the concentrations or activities of endogenous or exogenous molecules, a histological specimen, a physical quantity, or an internal image. Some general requirements of biomarkers are that they objectively measure a continuously distributed variable using physical units of measurement; have a clear, well understood relationship with the pathology of interest; are sensitive to improvement to and worsening of clinical state; and can be measured with suitable accuracy and precision. Non-invasive or minimally invasive biomarkers are particularly desirable, as they promote patient comfort and minimally disturb the pathology of interest.
A goal for developing image-based biomarkers for muscle disease is to reflect muscle disease in ways that are complementary to, more specific than, more spatially selective than, and/or less invasive than existing approaches. One particular advantage of qMRI in this regard is that it has the potential to integrate multiple types of information and thus potentially characterize many aspects of the disease process. This ability is very important in muscle diseases, which frequently exhibit a spatially variable, complex pathology that includes inflammation, necrosis and/or atrophy with fat replacement, fibrosis, disruption of the myofilament lattice ("Z-disk streaming"), and membrane damage. Another advantage of qMRI methods is that qualitative or semi-quantitative descriptions of contrast-based MR images reflect not just pathology, but also differences in image acquisition parameters, hardware, and human perception. An example of this last issue was demonstrated by Wokke et al., who showed that semi-quantitative assessments of fat infiltration are highly variable and frequently incorrect, when compared with quantitative fat/water MRI (FWMRI)1.
The protocol described here includes pulse sequences for measuring the longitudinal (T1) and transverse (T2) relaxation time constants, quantitative magnetization transfer (qMT) parameters, water diffusion coefficients using diffusion tensor MRI (DT-MRI), and muscle structure using structural images and FWMRI. T1 is measured by using an inversion recovery sequence, in which the net magnetization vector is inverted and its magnitude is sampled as the system returns to equilibrium. T2 is measured by repeatedly refocusing transverse magnetization using a train of refocusing pulses, such as the Carr-Purcell Meiboom-Gill (CPMG) method, and sampling the resulting spin-echoes. T1 and T2 data can be analyzed using non-linear curve-fitting methods that either assume a number of exponential components a priori (typically between one and three) or by using a linear inverse approach which fits the observed data to the sum of a large number of decaying exponentials, resulting in a spectrum of signal amplitudes. This approach requires a non-negative least square (NNLS) solution3, and typically includes additional regularization to produce stable results. T1 and T2 measurements have been widely used to study muscle diseases and injury4-9. T1 values are typically decreased in fat-infiltrated regions of muscle and elevated in inflamed regions4-6; T2 values are elevated in both fat-infiltrated and inflamed regions10.
QMT-MRI characterizes the free water and solid-like macromolecular proton pools in tissues by estimating the ratio of macromolecular to free water protons (the pool size ratio, PSR); the intrinsic relaxation rates of these pools; and the rates of exchange between them. Common qMT approaches include pulsed saturation11 and selective inversion recovery12,13 methods. The protocol below describes use of the pulsed saturation approach, which exploits the broad linewidth of the macromolecular proton signal, relative to the narrow linewidth of the water proton signal. By saturating the macromolecular signal at resonance frequencies sufficiently different from the water signal, the water signal is reduced as a result of magnetization transfer between the solid and free water proton pools. The data are analyzed using a quantitative biophysical model. QMT has been developed and applied in healthy muscles14,15, and a recent abstract appeared describing its implementation in muscle disease16. QMT has been used to study small animal models of muscle inflammation, wherein it has been shown that inflammation decreases the PSR17. Inasmuch as MT reflects both macromolecular and water contents, MT data may also reflect fibrosis18,19.
DT-MRI is used to quantify the anisotropic diffusion behavior of water molecules in tissues with ordered, elongated cells. In DT-MRI, water diffusion is measured in six or more different directions; these signals are then fitted to a tensor model20. The diffusion tensor, D, is diagonalized to obtain three eigenvalues (which are the three principal diffusivities) and three eigenvectors (which indicate the directions corresponding to the three diffusion coefficients). These and other quantitative indices derived from D provide information about tissue structure and orientation at a microscopic level. The diffusion properties of muscle, especially the third eigenvalue of D and the degree of diffusion anisotropy, reflect muscle inflammation17 and muscle damage due to experimental injury21, strain injury22, and disease23,24. Other potential influences on the diffusion properties of muscle include changes in cell diameter25 and membrane permeability changes.
Lastly, muscle atrophy, without or without macroscopic fat infiltration, is a pathological component of many muscle diseases. Muscle atrophy can be evaluated by using structural images to measure muscle cross-sectional area or volume and FW-MRI to assess fatty infiltration. Fat infiltration can be qualitatively described in T1- and T2-weighted images26, but fat and water signals are best measured by forming images that exploit the different resonance frequencies of fat and water protons27-29. Quantitative fat/water imaging methods have been applied in muscle diseases such as muscular dystrophy1,30,31, and can predict the loss of ambulation in these patients31.
The qMRI protocol described here uses all of these measurements to characterize muscle condition in the autoimmune inflammatory myopathies dermatomyositis (DM) and polymyositis (PM). Further details of the protocol, including its reproducibility, have been published previously32. The protocol includes standard pulse sequences as well as radiofrequency (RF) and magnetic field gradient objects specifically programmed on our systems. The authors anticipate that the protocol is also applicable in other neuromuscular disorders characterized by muscle atrophy, inflammation, and fat infiltration (such as the muscular dystrophies).
Protokół
NOTE: The reader is reminded that all research involving human subjects must be approved by the local Institutional Review Board (IRB) for the Use of Human Subjects in Research. Research participants must be informed of the purpose, procedures, risks, and benefits of the proposed research; the availability of alternative treatments or procedures; the availability of remuneration; and of their rights to privacy and to withdraw their consent and discontinue their participation. Prior to the MRI testing session, an investigator must present a potential research participant with an IRB-approved informed consent document (ICD), explain its contents, and ask the potential research participant if he/she wishes to participate in the study. If so, the participant will have to sign and date the ICD prior to completing any of the steps of the protocol here.
1. Actions Prior to the Day of Testing
- Restrict Lifestyle Habits that Could Confound the Data
- Instruct the participant not to perform moderate or heavy exercise during the 48 h prior to testing. Instruct the participant to abstain from over-the-counter medication and alcohol intake during the 24 h prior to testing. Instruct the participant to refrain from tobacco use or caffeine consumption during the 6 h prior to testing.
- Prior to testing, confirm that the participant has been compliant with these instructions.
- Prepare the MRI System
- Ensure the availability of all necessary equipment, as listed in the Table of Materials and Equipment.
- Define an MRI protocol; suggested parameters are found in Tables 1 - 5.
2. Day of Testing: Prepare for MRI Data Acquisition
- Conduct Safety Screening
- Screen for potential hazards in the MRI environment by having an MRI safety-trained healthcare worker present the research participant with a suitable MRI safety form, such as that found at www.mrisafety.com.
- If there any implanted magnetic or magnetically sensitive objects, ensure that they are safe for MRI scanning.
- Prepare the MRI System
- Ensure that all personnel have removed all magnetic and magnetically sensitive objects before entering the room that houses the MRI system. Conduct this check every time that someone enters the MRI room.
- Prepare the MRI system by placing the receive coil on the patient bed of the MRI system. Also, place a mattress with sheet and pillow with pillowcase on the bed. Have straps available to place around the thighs and bolsters or pillows to place under the knees.
- Start the software interface, enter patient data, and open the imaging protocol.
- Position the Research Participant on the MRI Scanner Table
- Observe the research participant as he/she checks his/her person and clothing for magnetically sensitive objects. Secure these objects outside of the MRI room in a lockable container. Enter the MRI room with the research participant immediately after completing this step.
- Position the participant on the patient bed in a supine, feet-first position. Place the body part to be imaged as close to the midline of the table as practical. Place bolsters or pillows under the knees to provide strain relief for the lower back and place a pillow under the head. To limit motion, gently but effectively secure the thigh, leg, and feet and ensure that the participant is comfortable.
- Place the RF receiver coil around the participant's thighs and connect it to the MRI system.
- Instruct the Participant and Complete Final Pre-testing Steps
- Give instructions about how to communicate with the investigators. Provide the participant with hearing protection and a signaling device that can be used to call for attention if needed. Instruct the participant of the need to stay still during and between all imaging sequences.
- Advance the patient bed into the MRI scanner such that the body part to be imaged is aligned to the center of the MRI scanner.
- After exiting the MRI room, confirm that the patient communication system is working and see that the participant is comfortable. Throughout the protocol, communicate regularly with the participant to ensure his/her comfort and compliance with instructions.
3. Day of Testing: Acquire the MRI Data
- Preparatory Steps
- As the MRI system determines the instrumental settings and calibrations prior to each imaging sequence (center frequency, receiver gain calibration, etc.), supervise these processes and ensure that each step is being performed correctly.
- Using a suitable software interface, acquire a set of localizer images (also known as pilot or scout images); using suggested parameters presented in Table 2.
- Determine where to place the center slice for qMRI data acquisitions, by identifying areas of damage and/or by referencing the slice position relative to reproducible anatomical landmarks.
- Transmit and Receive Coil Calibration Steps
- For these steps as well as all of the subsequent imaging steps, define region of anatomy in which to optimize the homogeneity of the static magnetic field (B0), a process known as "shimming". See Figure 1A for the typical placement of the shimming volume of interest (VOI) used in the present studies.
- If the MRI scanner has a multi-element transmission coil, acquire an RF calibration dataset.
- If the MRI scanner has a multi-element receive coil, acquire a spatial sensitivity map of the coils.
- Acquire Structural MRI Data
- Acquire high resolution, multi-slice, T1-weighted images using a fast spin-echo (FSE) sequence; the imaging parameters used in the present studies are provided in Table 1.
- Acquire high resolution, multi-slice, T2-weighted images using an FSE sequence; the imaging parameters used in the present studies are provided in Table 2.
- Acquire Data for Real-time Quality Control and Making Post-processing Corrections
- Acquire three-dimensional (3D) multiple gradient-echo data for the calculation of B0 field maps. The imaging parameters used in the present studies are provided in Table 3.
- Examine the field maps to ensure that there are no deviations of greater than ±60 Hz (approximately 0.5 parts per million at 3 Tesla) across the image. If there are, adopt an alternative approach to shimming (different method, different placement of VOI, etc.).
- Acquire 3D data for the calculation of nutation angle maps. The imaging parameters used in the present studies are provided in Table 2.
- Examine the field maps to ensure that there are no areas that deviate excessively from the nominal nutation angle. For the RF pulses that are used in this protocol, deviations greater than ± 30% of the nominal nutation angle are considered excessive.
- Acquire the qMRI Data
- Acquire 3D images for calculation of the T1, using an inversion recovery sequence. The imaging parameters used in the present studies are presented in Table 3.
- Repeat the T1 measurement in the presence of fat signal suppression (FS; this parameter is abbreviated T1,FS).
- Acquire single-slice images for calculation of the T2, using a multiple spin-echo sequence. Use the imaging parameters presented in Table 3.
- Repeat the T2 measurement in the presence of FS (T2,FS).
- Acquire 3D images for calculation of qMT parameters, using a pulsed saturation sequence with FS and the imaging parameters given in Table 4.
- Acquire multi-slice data for calculation of diffusion-tensor parameters, using a series of diffusion-weighted images. The imaging parameters used in these studies are given in Table 4.
- Acquire 3D data for calculation of fat/water images, using a series of six gradient-echo images. The imaging parameters used in these studies are given in Table 5.
- After Completing the qMRI Protocol
- Ensure that all images are of suitable quality by examining them for potentially correctable artifacts and by measuring the sufficient signal-to-noise ratio.
- For each qMRI dataset, define several regions of interest (ROIs) in the image series and examine the signal as a function of the relevant parameter (for example, for the T1-dependent data acquired in steps 3.5.1 and 3.5.2, plot the signal as a function of TI and ensure that the data follow the inversion-recovery function listed below in step 4.1.2).
- After completing a personal screening for magnetically sensitive objects, enter the MRI room. Remove the participant from the magnet, remove all straps and padding, and assist the participant in exiting the MRI scanner and the MRI room.
- Transfer the data, using methods compliant with local health privacy laws, to a local workstation for processing; data may be exported as Digital Imaging Communications in Medicine (DICOM) files or in the vendor's proprietary format (the method used in this protocol).
4. Analyze the qMRI Data
- Calculate the Parametric Maps
- Use a computer program designed for scientific computing and image analysis. By examining a histogram of the signal intensities in the image, form a signal threshold-based image mask that delineates areas of signal from areas of noise. Complete the steps below for every pixel in the signal portions of the images.
- Analyze the T1 data by measuring the signal intensity S for each inversion time (TI). Then, fit the values for S to an inversion-recovery with reduced pre-delay model:
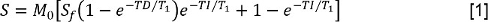
where M0 is a signal intensity representing the magnetization at the equilibrium state, Sf is the inversion ratio, and TD is the pre-delay time. Then, fit the data with FS to the same model, allowing determination of the longitudinal relaxation time constant with FS, T1,FS. - Analyze theT2 data by measuring S at each TE. Then, fit the data to a mono-exponential decay model:

where N is the signal offset at baseline. The reader may also decide to fit the data to a multi-exponential model, such as that below:

where J is the number of exponential components and f and T2,j are the signal fraction and T2 values associated with the jth component. Or, the reader may use a non-negative least squares (NNLS) method3. In the latter case, the Multi-exponential Relaxation Analysis (MERA) toolbox33 is freely available; other programs are available too. Repeat these analyses for the data with and without FS. - To analyze the qMT data, measure S for each irradiation power and frequency offset. Correct the nominal irradiation powers (represented by ω1 in the equation below) using the nutation angle maps. Correct the frequency offsets (Δf in the equation below) by using the B0 maps to adjust the applied offset frequencies. Then, fit the data to the following model34,35
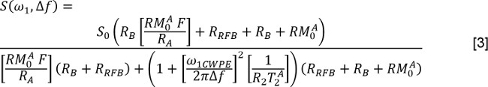
where is the exchange rate from the macromolecular pool to the free water pool, is the longitudinal relaxation rate of the free water pool, is the longitudinal relaxation rate of the macromolecular pool (assumed to be 1 s-1), is the PSR, is the T2 of the free water pool, and ω1CWPE is the average power of the saturation pulse. The saturation rate of the longitudinal magnetization of the macromolecular pool, is described by a super-Lorentzian model, as described in work by Henkelman and colleagues34,35. - To analyze the DTI data, first use an affine transformation algorithm36 to register each diffusion-weighted image to the corresponding non-diffusion weighted image. Then, for each pixel, measure the values for S in the non-diffusion weighted image and in each diffusion-weighted direction. Form a matrix composed of the diffusion encoding directions. Using multivariate, weighted least squares regression, regress the signal data on the diffusion encoding matrix and form D. Diagonalize D and perform a magnitude-sorting of the eigenvalues and their eigenvectors. Then calculate the mean diffusivity (MD) as:

where λ1, λ2, and λ3 are the eigenvalues of the diffusion tensor. Also calculate the fractional anisotropy (FA) as:

- Analyze the FWMRI data using a quantitative approach that separates water and fat signals based on chemical shift (such as the FattyRiot algorithm, available for free download from https://github.com/welcheb/FattyRiot).
- Define Regions of Interest for Analysis
- Specify ROIs on the anatomical images (by defining the boundaries of each muscle of interest). An example is shown in Figure 1.
- Resize the ROIs to match the matrix size of the qMRI images. As necessary, adjust the alignment of the ROIs to match the qMRI map (for example, if the participant moved between acquisitions, a translation of the ROI position might be required to avoid overlapping the muscle boundaries).
- Examine each ROI. If necessary, ensure that no pixels are included that contain partial volume artifacts, non-contractile tissue, and flow artifacts; please see Figure 1 for examples.
- Calculate the mean and standard deviation of the qMRI values in all pixels within the selected ROIs.
Wyniki
Figure 1 shows representative axial anatomical images acquired at the mid-thigh of a patient with polymyositis. Also shown is the location of the in-plane projection of the shim volume. Representative parameter maps for each qMRI method, all obtained from this same patient, are provided from Figures 2 - 7.
Figures 2A and 2B show the ΔB0 ...
Dyskusje
Muscle diseases such as the muscular dystrophies and idiopathic inflammatory myopathies constitute of group of diseases that are heterogeneous in etiology and, as individual entities, rare in their incidence. For example, Duchenne muscular dystrophy — the most common form of muscular dystrophy — has an incidence of 1 in 3,500 live male births37,38; dermatomyositis, to which this protocol has been applied, has an incidence of 1 in 100,00039. The higher collective incidence of these diseas...
Ujawnienia
None of the authors has a financial conflict of interest to report.
Podziękowania
We acknowledge grant support from the National Institutes of Health: NIH/NIAMS R01 AR050101 (BMD), NIH/NIAMS R01 AR057091 (BMD/JHP), NIH/NIBEB K25 EB013659 (RDD), and the Vanderbilt CTSA award RR024975. We also thank the reviewers for the comments and the subject for participating in these studies.
Materiały
| Name | Company | Catalog Number | Comments |
| 3T human MRI system | Philips Medical Systems (Best, the Netherlands) | Achieva/Intera | |
| Cardiac phased array receive coil | Philips Medical Systems | ||
| Pillows, straps, bolsters, and other positioning devices | |||
| Computer with MATLAB software | The Mathworks, Inc (Natick, MA) | r. 2014 |
Odniesienia
- Wokke, B. H., et al. Comparison of Dixon and T1-weighted MR methods to assess the degree of fat infiltration in duchenne muscular dystrophy patients. J Magn Reson Imaging. 38 (3), 619-624 (2013).
- Carr, H., Purcell, E. Effects of diffusion on free precession in NMR experiments. Phys Rev. 94, 630-638 (1954).
- Whittall, K. P., MacKay, A. L. Quantitative interpretation of NMR relaxation data. Journal of Magnetic Resonance. 84 (1), 134-152 (1989).
- Park, J. H., et al. Dermatomyositis: correlative MR imaging and P-31 MR spectroscopy for quantitative characterization of inflammatory disease. Radiology. 177 (2), 473-479 (1990).
- Park, J. H., et al. Magnetic resonance imaging and p-31 magnetic resonance spectroscopy provide unique quantitative data useful in the longitudinal management of patients with dermatomyositis. Arthritis & Rheumatism. 37 (5), 736-746 (1994).
- Park, J. H., et al. Use of magnetic resonance imaging and p-31 magnetic resonance spectroscopy to detect and quantify muscle dysfunction in the amyopathic and myopathic variants of dermatomyositis. Arthritis & Rheumatism. 38 (1), 68-77 (1995).
- Huang, Y., et al. Quantitative MR relaxometry study of muscle composition and function in Duchenne muscular dystrophy. J Magn Reson Imaging. 4 (1), 59-64 (1994).
- Kim, H. K., et al. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology. 255 (3), 899-908 (2010).
- Arpan, I., et al. T2 mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5-15-year-old boys with Duchenne muscular dystrophy. NMR in Biomedicine. 26 (3), 320-328 (2013).
- Fan, R. H., Does, M. D. Compartmental relaxation and diffusion tensor imaging measurements in vivo in λ-carrageenan-induced edema in rat skeletal muscle. NMR in Biomedicine. 21 (6), 566-573 (2008).
- Sled, J. G., Pike, G. B. Quantitative interpretation of magnetization transfer in spoiled gradient echo MRI sequences. J Magn Reson. 145 (1), 24-36 (2000).
- Gochberg, D. F., Gore, J. C. Quantitative magnetization transfer imaging via selective inversion recovery with short repetition times. Magn Reson Med. 57 (2), 437-441 (2007).
- Li, K., et al. Optimized inversion recovery sequences for quantitative T1 and magnetization transfer imaging. Magn Reson Med. 64 (2), 491-500 (2010).
- Louie, E. A., Gochberg, D. F., Does, M. D., Damon, B. M. Magnetization transfer and T2 measurements of isolated muscle: effect of pH. Magn Reson Med. 61 (3), 560-569 (2009).
- Sinclair, C. D. J., et al. Quantitative magnetization transfer in in vivo healthy human skeletal muscle at 3 T. Magn Reson Med. 64 (6), 1739-1748 (2010).
- Sinclair, C., et al. Multi-parameter quantitation of coincident fat and water skeletal muscle pathology. Proc 21st Ann Meeting ISMRM. , (2013).
- Bryant, N., et al. Multi-parametric MRI characterization of inflammation in murine skeletal muscle. NMR Biomed. 27 (6), 716-725 (2014).
- Aisen, A. M., Doi, K., Swanson, S. D. Detection of liver fibrosis with magnetic cross-relaxation. Magn Reson Med. 31 (5), 551-556 (1994).
- Kim, H., et al. Induced hepatic fibrosis in rats: hepatic steatosis, macromolecule content, perfusion parameters, and their correlations-preliminary MR imaging in rats. Radiology. 247 (3), 696-705 (2008).
- Basser, P. J., Mattiello, J., LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys J. 66 (1), 259-267 (1994).
- Heemskerk, A., Strijkers, G., Drost, M., van Bochove, G., Nicolay, K. Skeletal muscle degeneration and regeneration following femoral artery ligation in the mouse: diffusion tensor imaging monitoring. Radiology. 243 (2), 413-421 (2007).
- Zaraiskaya, T., Kumbhare, D., Noseworthy, M. D. Diffusion tensor imaging in evaluation of human skeletal muscle injury. J Magn Reson Imaging. 24 (2), 402-408 (2006).
- Qi, J., Olsen, N. J., Price, R. R., Winston, J. A., Park, J. H. Diffusion-weighted imaging of inflammatory myopathies: polymyositis and dermatomyositis. J Magn Reson Imaging. 27 (1), 212-217 (2008).
- McMillan, A. B., Shi, D., Pratt, S. J., Lovering, R. M. Diffusion tensor MRI to assess damage in healthy and dystrophic skeletal muscle after lengthening contractions. J Biomed Biotech. , (2011).
- Scheel, M., et al. Fiber type characterization in skeletal muscle by diffusion tensor imaging. NMR Biomed. 26 (10), 1220-1224 (2013).
- Kaufman, L. D., Gruber, B. L., Gerstman, D. P., Kaell, A. T. Preliminary observations on the role of magnetic resonance imaging for polymyositis and dermatomyositis. Annalsrheumatic Dis. 46 (8), 569-572 (1987).
- Dixon, W. T. Simple proton spectroscopic imaging. Radiology. 153 (1), 189-194 (1984).
- Glover, G. H. Multipoint Dixon technique for water and fat proton and susceptibility imaging. J Magn Reson Imaging. 1 (5), 521-530 (1991).
- Berglund, J., Kullberg, J. Three-dimensional water/fat separation and T2* estimation based on whole-image optimization--application in breathhold liver imaging at 1.5 T. Magn Reson Med. 67 (6), 1684-1693 (2012).
- Gloor, M., et al. Quantification of fat infiltration in oculopharyngeal muscular dystrophy: Comparison of three MR imaging methods. J Magn Reson Imaging. 33 (1), 203-210 (2011).
- Fischmann, A., et al. Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J Neurol. 260 (4), 969-974 (2013).
- Li, K., et al. Multi-parametric MRI characterization of healthy human thigh muscles at 3.0 T - relaxation, magnetization transfer, fat/water, and diffusion tensor imaging. NMR Biomed. 27 (9), 1070-1084 (2014).
- Morrison, C., Stanisz, G., Henkelman, R. M. Modeling magnetization transfer for biological-like systems using a semi-solid pool with a super-Lorentzian lineshape and dipolar reservoir. J Magn Reson Series B. 108 (2), 103-113 (1995).
- Li, J. G., Graham, S. J., Henkelman, R. M. A flexible magnetization transfer line shape derived from tissue experimental data. Magn Reson Med. 37 (6), 866-871 (1997).
- Mangin, J. F., Poupon, C., Clark, C., Le Bihan, D., Bloch, I. Distortion correction and robust tensor estimation for MR diffusion imaging. Med Image Anal. 6 (3), 191-198 (2002).
- Moser, H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum Genet. 66 (1), 17-40 (1984).
- van Essen, A. J., Busch, H. F., te Meerman, G. J., ten Kate, L. P. Birth and population prevalence of Duchenne muscular dystrophy in The Netherlands. Hum Genet. 88 (3), 258-266 (1992).
- Bendewald, M. J., Wetter, D. A., Li, X., Davis, M. P. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: A population-based study in olmsted county, minnesota. Arch Dermatol. 146 (1), 26-30 (2010).
- Carlier, P. G. Global T2 versus water T2 in NMR imaging of fatty infiltrated muscles: different methodology, different information and different implications. Neuromuscul Disord. 24 (5), 390-392 (2014).
- Foley, J. M., Jayaraman, R. C., Prior, B. M., Pivarnik, J. M., Meyer, R. A. MR measurements of muscle damage and adaptation after eccentric exercise. J Appl Physiol. 87 (6), 2311-2318 (1999).
- Garrood, P., et al. MR imaging in Duchenne muscular dystrophy: quantification of T1-weighted signal, contrast uptake, and the effects of exercise. J Magn Reson Imaging. 30 (5), 1130-1138 (2009).
- Bratton, C. B., Hopkins, A. L., Weinberg, J. W. Nuclear magnetic resonance studies of living muscle. Science. 147, 738-739 (1965).
- Fleckenstein, J. L., Canby, R. C., Parkey, R. W., Peshock, R. M. Acute effects of exercise on MR imaging of skeletal muscle in normal volunteers. AJR Am J Roentgenol. 151 (2), 231-237 (1988).
- Williams, S., Heemskerk, A., Welch, E., Damon, B., Park, J. The quantitative effects of inclusion of fat on muscle diffusion tensor MRI measurements. J Magn Reson Imaging. 38 (5), 1292-1297 (2013).
- Hernando, D., et al. Removal of olefinic fat chemical shift artifact in diffusion MRI. Magn Reson Med. 65 (3), 692-701 (2011).
- Willcocks, R. J., et al. Longitudinal measurements of MRI-T2 in boys with Duchenne muscular dystrophy: effects of age and disease progression. Neuromuscul Disord. 24 (5), 393-401 (2014).
- Poon, C. S., Henkelman, R. M. Practical T2 quantitation for clinical applications. J Magn Reson Imaging. 2 (5), 541-553 (1992).
- Does, M. D., Gore, J. C. Complications of nonlinear echo time spacing for measurement of T2. NMR Biomed. 13 (1), 1-7 (2000).
- Poon, C. S., Henkelman, R. M. 180° refocusing pulses which are insensitive to static and radiofrequency field inhomogeneity. J Magn Reson. 99 (1), 45-55 (1992).
- Hollingsworth, K. G., de Sousa, P. L., Straub, V., Carlier, P. G. Towards harmonization of protocols for MRI outcome measures in skeletal muscle studies: consensus recommendations from two TREAT-NMD NMR workshops, 2 May 2010, Stockholm, Sweden, 1-2 October 2009, Paris, France. Neuromuscul Disord. 22, S54-S67 (2010).
- Underhill, H. R., Rostomily, R. C., Mikheev, A. M., Yuan, C., Yarnykh, V. L. Fast bound pool fraction imaging of the in vivo rat brain: Association with myelin content and validation in the C6 glioma model. Neuroimage. 54 (3), 2052-2065 (2011).
- Smith, S. A., et al. Quantitative magnetization transfer characteristics of the human cervical spinal cord in vivo: application to adrenomyeloneuropathy. Magn Reson Med. 61 (1), 22-27 (2009).
- Li, K. D. R., Dortch, R. D., Gochberg, D. F., Smith, S. A., Damon, B. M., Park, J. H. Quantitative magnetization transfer with fat component in human muscles. Proc. 20th Ann Meeting ISMRM. , (2012).
- Damon, B. M. Effects of image noise in muscle diffusion tensor (DT)-MRI assessed using numerical simulations. Magn Reson Med. 60 (4), 934-944 (2008).
- Damon, B. M., Buck, A. K. W., Ding, Z. Diffusion-tensor MRI-based skeletal muscle fiber tracking. Imaging Med. 3 (6), 675-687 (2011).
- Froeling, M., Nederveen, A. J., Nicolay, K., Strijkers, G. J. DTI of human skeletal muscle: the effects of diffusion encoding parameters, signal-to-noise ratio and T2 on tensor indices and fiber tracts. NMR in Biomedicine. 26 (11), 1339-1352 (2013).
- Basser, P. J., Pajevic, S. Statistical artifacts in diffusion tensor MRI (DT-MRI) caused by background noise. Magn Reson Med. 44 (1), 41-50 (2000).
- Anderson, A. W. Theoretical analysis of the effects of noise on diffusion tensor imaging. Magn Reson Med. 46 (6), 1174-1188 (2001).
- Saupe, N., White, L. M., Stainsby, J., Tomlinson, G., Sussman, M. S. Diffusion tensor imaging and fiber tractography of skeletal muscle: optimization of B value for imaging at 1.5 T. AJR Am J Roentgenol. 192 (6), W282-W290 (2009).
- Levin, D. I., Gilles, B., Madler, B., Pai, D. K. Extracting skeletal muscle fiber fields from noisy diffusion tensor data. Med Image Anal. 15 (3), 340-353 (2011).
- Sinha, U., Sinha, S., Hodgson, J. A., Edgerton, R. V. Human soleus muscle architecture at different ankle joint angles from magnetic resonance diffusion tensor imaging. J Appl Physiol. 110 (3), 807-819 (2011).
- Jones, D. K., Cercignani, M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 23 (7), 803-820 (2010).
- Hamilton, G., et al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 24 (7), 784-790 (2011).
- Hernando, D., Kellman, P., Haldar, J. P., Liang, Z. P. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med. 63 (1), 79-90 (2010).
- Hernando, D., Hines, C. D., Yu, H., Reeder, S. B. Addressing phase errors in fat-water imaging using a mixed magnitude/complex fitting method. Magn Reson Med. 67 (3), 638-644 (2012).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone