Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Transvaginal Mesh Insertion in the Ovine Model
W tym Artykule
Podsumowanie
This protocol describes mesh implantation in the ovine rectovaginal septum using a single vaginal incision technique, with and without the trocar-guided insertion of anchoring arms.
Streszczenie
This protocol describes mesh insertion into the rectovaginal septum in sheep using a single vaginal incision technique, with and without the trocar-guided insertion of anchoring arms. Parous sheep underwent the dissection of the rectovaginal septum, followed by the insertion of an implant with or without four anchoring arms, both designed to fit the ovine anatomy. The anchoring arms were put in place using a trocar and an "outside-in" technique. The cranial arms were passed through the obturator, gracilis, and adductor magnus muscles. The caudal arms were fixed near the sacrotuberous ligament, through the coccygeus muscles. This technique allows for the mimicking of surgical procedures performed in women suffering from pelvic organ prolapse. The anatomical spaces and elements are easily identified. The most critical part of the procedure is the insertion of the cranial trocar, which can easily penetrate the peritoneal cavity or the surrounding pelvic organs. This can be avoided by a more extensive retroperitoneal dissection and by guiding the trocar more laterally. This approach is designed only for experimental testing of novel implants in large animal models, as trocar-guided insertion is currently not used clinically.
Wprowadzenie
Pelvic organ prolapse is clinically diagnosed in half of women who had at least one vaginal delivery, but subjectively, it bothers half of women overall1. The mainstay of therapy is surgical reconstruction using either native tissue or implant materials, but each of these methods has its limitations, including recurrence or local complications2,3,4. The ideal implant has not yet been identified; hence, there is an ongoing demand for product innovation and for the development of a proper pipeline for preclinical experimentation prior to the introduction of new products and techniques to the market. One of the steps in this track is experimental evaluation on suitable animal models5,6. Ideally, they should mimic the anatomical, biomechanical, and biological environments. When it comes to the experimental evaluation of novel implants, they are typically tested first in smaller models, either for biocompatibility or for the reconstruction of abdominal wall defects. That type of experiments has been criticized, because the implants are not inserted into the area of interest (i.e., the vagina)7. Vaginal surgery models are more scarce, certainly when the goal of the experiment is to document the biomechanical characteristics of explants. For this reason, there was a move from rabbits to sheep8. Adult ewes are large-animal models with a reasonably sized and accessible vagina. They can be used for the mid-term evaluation of novel implants, and it is possible to reproduce vaginal exposures with certain materials9,10,11,12,13. Not only the dimensions and anatomy of the ovine vagina and pelvic floor are comparable to those in humans, but also the spontaneous occurrence of prolapse, which occurs in 15% of ewes. Prolapse risk factors are overlapping (i.e., multiparity, previous history of POP, increased intra-abdominal pressure induced by a higher bodyweight or when grazing on hills, and comparable effects of (phyto)estrogens)6,14. In Europe, sheep are the only reasonable alternative, as research on non-human primates has been nearly completely banned. Here, the model was taken one step further by mimicking the transvaginal insertion of implants using trocars and guides for the tension-free placement of meshes into the recto-vaginal septum. This was followed by fixing the implant using anchoring with arms through the ligaments of muscles, which can be considered equivalent to clinical practice15,16. So far, this technique has not been studied, though many believe that specific complications may occur due to the use of these longer strips and/or the piercing of anatomical structures.
In an earlier detailed anatomical study, the ovine pelvic floor was compared to the female pelvis17. When it comes to anchoring the implant, sheep do not have the sacrospinous ligament, yet they do have a very well-developed and broad sacrotuberous ligament. The pudendal nerve runs ventrally over it, making it unsafe to use this landmark as a suspension point. Conversely, the coccygeus muscle and its fascia, as well as the obturator membrane, are accessible through the rectovaginal space. Here, the access and position of the anatomical structures for the fixation of anchoring arms is proposed. The instruments that can be used to position the mesh are discussed. Finally, the relationship of the arms or trocars to adjacent anatomical structures, such as vessels and nerves, as well as potential intraoperative complications, are also described.
Protokół
Ethical approval for this experiment was obtained from the Ethics Committee on Animal Experimentation of the KU Leuven (P065/2013). Animals were treated in accordance with current national guidelines on animal welfare.
1. Material and the Experimental Animal
- Surgery preparation
- In the surgical theater cover a table with a sterile drape and prepare one sterile curved trocar (Figure 1, panel A), sterile surgical instruments, sutures, and sterile gauze. Perform the entire surgical procedure in sterile conditions if the experiment includes follow-up. Place all instruments on the table to be ready for use during surgery.
- Remove a sterile rectangular implant and/or an implant with anchoring arms from the sterile package and put them on the table covered with sterile drape (Figure 1B, C, and D).
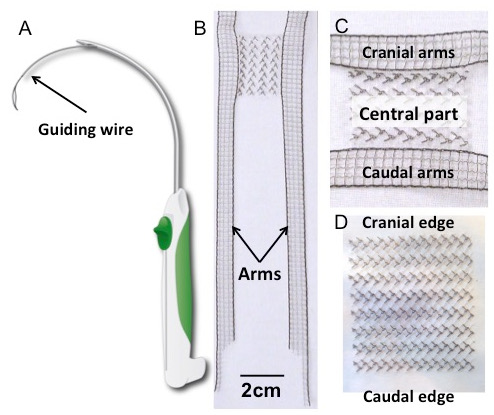
Figure 1: Trocar and Implants. (A) Schematic drawing of the trocar. (B) H-shaped polyvinylidene fluoride (PVDF) implant, with a detail of the central part (panel C). Its shape was inspired by the four-arm meshes currently available for transvaginal prolapse repair. The rectangular body (30 x 40 mm2) is laterally extended by four outstretched arms (150 x 10 mm2). The dimensions of the arms are designed to be long enough to pierce the relevant suspension structures, based on earlier anatomical studies17. (D) The rectangular implant (30 x 40 mm2). Both implants were made of polyvinylidene fluoride; textile characteristics and properties are in Table 1.
- Experimental animal (Ewe, 45-60 kg)
- Administer premedication of 1 mL of 15 mg/mL atropine sulfate and 1 mL/50 kg of xylazine HCl intramuscularly (i.m.) 30 min before the surgical procedure.
- After 30 min, ensure that the premedication has made the sheep lethargic and sleepy.
- Insert an intravenous catheter into the jugular vein and administer 0.075 mL/kg of ketamine 100 mg/mL HCl. Confirm deep anesthesia by observing the lack of reaction to painful stimuli.
- Move the animal onto the surgical table and secure its airways by intubation. Maintain the anesthesia with 2.5% isoflurane in 5 L/min oxygen.
- Keep the intravenous line inserted in the jugular vein and supply 500 mL of saline solution at a flow rate of 150 mL/h.
- Administer prophylactic antibiotics i.m. (amoxicillin clavulanate, 7 mg/kg) and post-operative analgesics (buprenorfin and chlorocresol, 1 mL) or the equivalent according to local protocols.
- Place the animal in lithotomy position on the end of the surgical table and secure its limbs, with the hips in hyper-flexion, using ropes (Figure 2, panel A).
- Manually empty the bladder and rectum by pushing on them trans-vaginally.
- Shave the perineum, the medial part of the thigh, and the tail folds and disinfect with polyvidone iodium 7.5% (Figure 2, panel B and C).
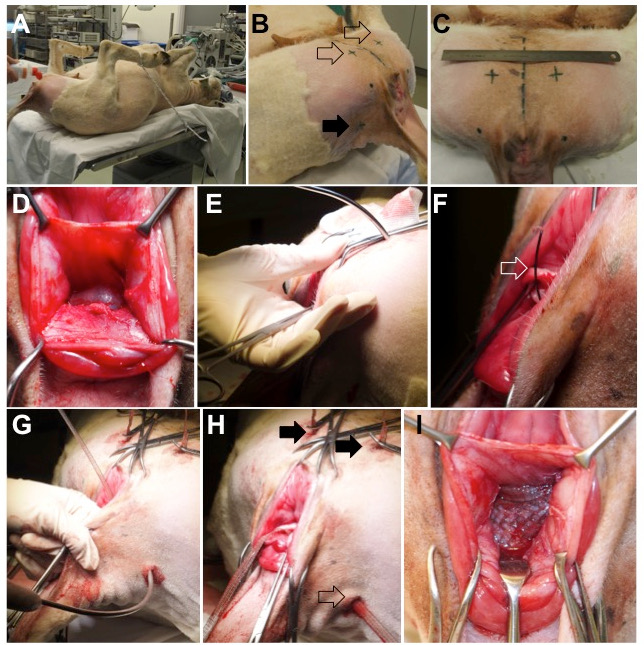
Figure 2: Animal Surgery. (A) A sheep placed in the supine position, with the hips hyper-flexed by securing the lower limbs. (B) The external entrance points for trocar insertion are on the ventral side (empty arrow) and dorsally on the lateral tail folds (full arrow). (C) Position of the ventral insertion points; the dashed line in the middle represents the midsagittal plane of the animal. (D) Dissected rectovaginal septum. (E) Insertion of the ventral trocar through the muscles on the medial side of the thigh, the obturator foramen, and the paravaginal space. The trajectory of the piercing trocar is controlled with the finger. (F and G) Once the trocar is in place, the wire sling (open arrow) is advanced and loaded with the arm of the vaginal mesh. (H) Final position of the ventral (full arrows) and dorsal (empty arrow) arms. (I) The central part is placed tension-free between the vaginal wall and the rectal adventitia.
- Prepare personnel for a surgery in sterile conditions. Put on a surgical cap and mouth mask, wash hands for surgery, and put on a surgical gown and sterile gloves.
- Cover the animal with a sterile drape and make an opening above the genital hiatus.
2. Surgical Procedure
- Preparation of the rectovaginal septum
- Grasp the dorsal vaginal wall 3 cm cranial to the hymeneal ring using Allis forceps.
- Take a syringe loaded with 10 mL of saline and fitted with a 22 G needle. Insert it through the vaginal epithelium (approximately 3 - 4 mm deep) and into the midline of the rectovaginal septum, 1.5 cm cranial to the hymeneal ring.
- Perform "aqua-dissection" by injecting saline in the rectovaginal septum11.
- Make a 3 cm-long midline incision on the vaginal epithelium, starting caudal to the Allis forceps (step 2.1.1) and ending at the hymeneal ring using a scalpel. Enter the recto-vaginal space through this incision.
- Place the self-retaining retractor (see the Table of Materials) over the perineum and place four sharp stay hooks in the vaginal incision to keep it open.
- With your finger, bluntly dissect the recto-vaginal fascia from the vaginal wall laterally towards the pelvic side walls and cranially up to the caudal aspect of the cul-de-sac. Create suitable space for the 30 x 40 mm2 central part of the mesh (Figure 2, panel D).
- Perform haemostasis with haemostatic forceps or a crisscross haemostatic ligature whenever necessary.
NOTE: Small bleeders can be clamped with the hemostatic forceps. This crushes the vessel and initiates the natural coagulation cascade. For stronger bleeding, grasp the bleeding vessel with forceps and place a crisscross ligature, securing it with a square knot. At this point, one can either insert the rectangular implant (step 2.2) or continue with the dissection to insert the implant with anchoring arms (step 2.3).
- Flat mesh insertion
- Insert the vaginal retractor into the vaginal incision to allow a better view of the cranial part of the dissected area.
- Suture the left and right cranial corner of the implant with a simple interrupted 3/0 polypropylene suture on the left and right sides of the most cranial aspect of the dissected recto-vaginal space. Cut the residual suture material. Keep the suture away from the vaginal lumen (i.e., do not penetrate the vaginal wall).
NOTE: The implant is always sutured to the connective tissue comprising the recto-vaginal septum. The vaginal wall is not penetrated if the suture material cannot be seen in the vagina. - Add one additional simple interrupted suture midway along the cranial aspect of the implant.
- Suture the lateral edges of the implant midway onto the surrounding connective tissue with a simple interrupted 3/0 polypropylene. Keep the implant as flat as possible and tension-free.
- Suture the left and right caudal corners with simple interrupted 3/0 polypropylene sutures on the left and right sides of the most caudal aspect of the rectovaginal space.
- Add one additional simple interrupted suture midway along the caudal aspect of the implant.
- Close the vaginal incisions with a running 3/0 polyglactin suture.
- Insertion and anchoring of implant with arms (trocar-guided technique).
- Continue the dissection of the recto-vaginal space created in step 2.1 cranio-ventrally to reach the medial aspect of the obturator foramen, which can easily be palpated.
- Dissect the space caudo-laterally to reach the caudal aspect of the sacrotuberous ligament and the caudally located coccygeus muscle.
- With a no. 24 blade, make four 1 cm-wide incisions on the vulvar side, cutting through the skin and superficial muscular fascia (Figure 2, panel B and C).
- Make two “ventral” incisions on the medial aspect of the thigh, proximately 4 cm cranial from the caudal border of the sciatic arch (i.e., the inferior border of the symphysis) and 3 cm lateral from the midline (Figure 2, panel C).
- Make two "dorsal" incisions at the level of the insertion of the tail folds, 2 cm medial to the tuber ischiadicum, which can be easily palpated (Figure 2, panel B).
- Place a curved trocar through one of the ventral incisions (Figure 2, panel E).
- Pass the trocar through the adductor magnus muscle, the external obturator, and the medial aspect of the obturator foramen.
- Control the progression of the trocar with a finger inserted through the vaginal incision. Guide its tip to the tendinous arc of the levator ani muscle (Figure 2, panel E).
- Expose the guiding wire in the vaginal wall incision and load it with the corresponding ipsilateral cranial mesh arm (Figure 2, panel F).
- Pull the trocar loaded with the mesh arm through the above structures. Keep the arm tension-free.
- Repeat the process with the second cranial arm through the ventral incision on the other side of the animal.
- Through one of dorsal incisions, pass the trocar through the coccygeus muscle, just distal to the sacrotuberous ligament (Figure 2, panel G).
- Expose the guide wire through the vaginal incision, grasp the dorsal arm of the mesh, and pull it out. Keep the arm tension-free and repeat on the other side.
- Adjust the position of the mesh by flattening it and applying tension to the arms, but keep the mesh tension-free (Figure 2, panel I; Figure 3).
- Fix the body of the mesh with a simple interrupted 3/0 polypropylene suture in the middle of its caudal border, securing it to the surrounding connective tissue.
- Cut the arms at the level of the skin and close all skin incisions with simple interrupted 3/0 polyglecaprone sutures (Figure 2, panel H).
- Close the vaginal incision with a running 3/0 polyglecaprone suture.
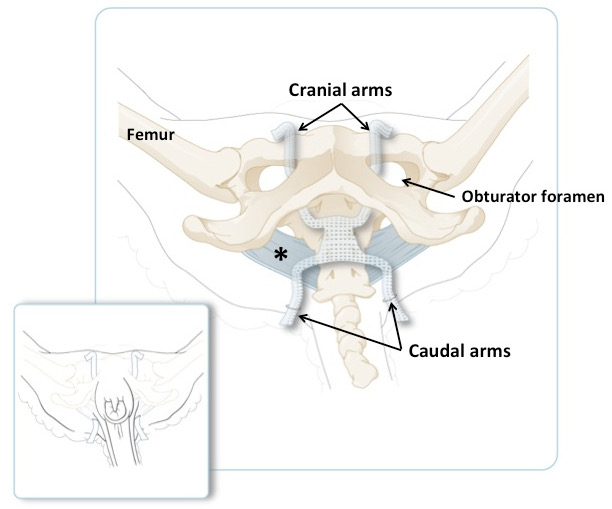
Figure 3: Schematic Illustration of the Ovine Pelvis, with the Cranial Arms Passing through the Obturator Foramen and the Caudal Arms Passing through the Tail Folds. The broad sacrotuberous ligament is in blue. The smaller panel illustrates the position of the arms on an animal in recumbent position, just before shortening the excessive amount of material. The main panel shows the same but with the skin and muscles removed.
Wyniki
Management in a Longer Observation Setup
Following the surgical procedure, vaginal packing (a saline-solution-soaked gauze package inserted in the vagina immediately after the surgery) may be inserted for 24 h to secure the implant position. The sheep should be placed in a recovery cage and its respiratory function followed until full recovery. Later, it is possible to place the sheep in the stable and to allow ...
Dyskusje
Here, we describe an experimental procedure in sheep, aimed to mimic vaginal dissection and transvaginal mesh insertion of an implant with or without anchoring arms. The subsequent steps and instruments were inspired by surgical procedures done for POP and stress urinary incontinence15,16,19,20. After initial anatomical dissections, there were still some problems during experimental mesh insert...
Ujawnienia
This research program on the ovine model was supported by an unconditional grant from Medri and Blasingame, Burch, Garrard and Ashley (Atlanta GA, USA). Agreements are handled via the Leuven Research and Development transfer office. Sponsors did not interfere with the planning, execution, or reporting of this experiment, nor do they own the results. NS and LH are recipients of a grant from the EC in the FP7-framework (Bip-Upy project; NMP3-LA-2012-310389). AF was supported by a grant from the EC in the industry-academic partnership program (251356).
Podziękowania
We thank Ivan Laermans, Rosita Kinart, Ann Lissens (Centre for Surgical Technologies, KU Leuven, Leuven, Belgium). Jo Verbinnen and Kristof Reyniers (Vesalius Institute of Anatomy, Faculty of Medicine, KU Leuven, Leuven, Belgium) provided technical support during the experiment. We thank Leen Mortier for the help with data and manuscript management. We thank FEG Textiltechniken for manufacturing prototype meshes, sterilizing them, and donating them unconditionally for research.
Materiały
| Name | Company | Catalog Number | Comments |
| Animals: | |||
| parous female sheep (45 - 65 kg) | Zoötechnical Institute of the KU Leuven | NA | experimetnal animal |
| Sterile clothing: | |||
| sterile drape 45 x 75 cm | Lohmann & Rauscher, Regensdorf, Germany | 33002 | other material |
| sterile OR drape 150 x 180 cm | Lohmann & Rauscher, Regensdorf, Germany | 33009 | other material |
| sterile glowes 2x | Lohmann & Rauscher, Regensdorf, Germany | 16652 | other material |
| sterile surgical gown 2x | Lohmann & Rauscher, Regensdorf, Germany | 19342 | other material |
| surgical head cap 2x | Lohmann & Rauscher, Regensdorf, Germany | 17427 | other material |
| surgical face mask 2x | Lohmann & Rauscher, Regensdorf, Germany | 11983 | other material |
| Other surgical material | |||
| implant | FEG Textiltechnik GmbH, Aachen, Germany | NA | purposely designed implant |
| 3/0 polypropylene suture | Prolene, Ethicon, Diegem, Belgium | 8762H | suture material |
| 3/0 polygecaprone suture | Vicryl, Ethicon | J311H | suture material |
| gauze swabs 10 x 10 cm 10x, 12-ply | Lohmann & Rauscher, Regensdorf, Germany | 11574 | other material |
| syringe 20 mL | Becton Dickinsosn S.A., Madrid, Spain | 300613 | aqua-dissection |
| needle 16 gauge | Terumo, Leuven, Belgium | NN-2238R | aqua-dissection |
| Surgical equipment: | |||
| blade no.22 | Fine science isntruments, Heidelberg, Germany | 10022-00 | surgical instruments |
| Allis tissue forceps 1x | Fine science isntruments, Heidelberg, Germany | 11091-15 | surgical instruments |
| Standart pattern forceps 1x2 theeth 1x | Fine science isntruments, Heidelberg, Germany | 11023-14 | surgical instruments |
| Standart pattern forceps straight serrated 1x | Fine science isntruments, Heidelberg, Germany | 11000-14 | surgical instruments |
| Scalpel handle 1x | Fine science isntruments, Heidelberg, Germany | 10004-13 | surgical instruments |
| Halstead-Mosquito forceps 2x | Fine science isntruments, Heidelberg, Germany | 13008-12 | surgical instruments |
| Standart pattern scissors 1x | Fine science isntruments, Heidelberg, Germany | 14001-14 | surgical instruments |
| Metzenbaum scissors 1x | Fine science isntruments, Heidelberg, Germany | 14016-18 | surgical instruments |
| Crile Wood needle holder 1x | Fine science isntruments, Heidelberg, Germany | 12003-15 | surgical instruments |
| Kell forceps 1x | Fine science isntruments, Heidelberg, Germany | 13018-14 | surgical instruments |
| Long Starr Self-Retaining Retractor with eight 5mm sharp stay hooks | Cooper Surgical, Tumbull, USA | 3704 | surgical instruments |
| Heaney Simon Vaginal Retractor | Medical supplies & equipments co., Katy, Texas, USA | 403-129FSI | surgical instruments |
| Trocar (Insnare) | Bard, West Sussex, United Kingdom | NA | any trocar on market for transvaginal mesh implantation |
| Medication: | |||
| amoxilicilline clavulanate 1000mg / 300 mL (Ampiciline) | GSK, Wavre, Belgium | NA | antibiotics |
| buprenorfin 0.3 mg/mL + chlorocresol 1.35 mg/mL (Vetregesic) | Ecuphar, Oostkamp, Belgium | NA | analgesia |
| ketamin HCL 100mg/mL (Ketamine 1000) | Ceva Sante Animale, Brussels, Belgium | NA | anesthesia |
| isoflurane (IsoFlo) | Abbott Laboratories Ltd, Maidenhead, Berkshire, UK | NA | anesthesia |
| polyvidone iodium 7.5% (Braunol) | B. Braun Medical, Machelen, Belgium | NA | local desinfection |
| saline solution 500ml | B. Braun Medical, Machelen, Belgium | NA | aqua-dissection |
| Xxylazine HCl , 1 mL/50 kg | Vexylan, Ceva Sante Animale, Belgium | NA | premedication |
| atropine Sulfate 15 mg/ml (), | Viatris, Belgium | NA | premedication |
Odniesienia
- Glazener, C., et al. Childbirth and prolapse: Long-term associations with the symptoms and objective measurement of pelvic organ prolapse. BJOG An Int. J. Obstet. Gynaecol. 120 (2), 161-168 (2013).
- Jia, X., et al. Efficacy and safety of using mesh or grafts in surgery for anterior and/or posterior vaginal wall prolapse: systematic review and meta-analysis. BJOG. 115 (11), 1350-1361 (2008).
- Maher, C., et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Review. 2 (2), 10-13 (2016).
- Nieminen, K., et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am. J. Obstet. Gynecol. 203 (3), e1-e8 (2010).
- Abramowitch, S. D., Feola, A., Jallah, Z., Moalli, P. A. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur. J. Obstet. Gynecol. Reprod. Biol. 144, S146-S158 (2009).
- Couri, B., Lenis, A., Borazjani, A., Paraiso, M. F. R., Damaser, M. S. Animal models of female pelvic organ prolapse: lessons learned. Expert Rev. Obs. Gynecol. 7 (3), 249-260 (2012).
- Deprest, J., et al. The biology behind fascial defects and the use of implants in pelvic organ prolapse repair. Int. Urogynecol. J. Pelvic Floor Dysfunct. 17, S16-S25 (2006).
- Ozog, Y., Mazza, E., De Ridder, D., Deprest, J. Biomechanical effects of polyglecaprone fibers in a polypropylene mesh after abdominal and rectovaginal implantation in a rabbit. Int. Urogynecol. J. 23 (10), 1397-1402 (2012).
- Manodoro, S., et al. Graft-related complications and biaxial tensiometry following experimental vaginal implantation of flat mesh of variable dimensions. BJOG. 120 (2), 244-250 (2013).
- Endo, M., et al. Cross-linked xenogenic collagen implantation in the sheep model for vaginal surgery. Gynecol. Surg. , 113-122 (2015).
- Feola, A., et al. Host reaction to vaginally inserted collagen containing polypropylene implants in sheep. Am. J. Obstet. Gynecol. 212 (4), e1-e474 (2015).
- Barnhart, K. T., et al. Baseline dimensions of the human vagina. Hum. Reprod. 21 (6), 1618-1622 (2006).
- Tayrac, R., Alves, A., Thérin, M. Collagen-coated vs noncoated low-weight polypropylene meshes in a sheep model for vaginal surgery. A pilot study. Int. Urogynecol. J. Pelvic Floor Dysfunct. 18 (5), 513-520 (2007).
- Sobiraj, A., Busse, G., I, H. B. O. S. E. D. Ivastigation into the blood plasma profiles progesterone in sheep sufferingform vaignal inversion and prolapse. Br. Vet. J. 142 (142), 218-223 (1986).
- Reisenauer, C., Kirschniak, A., Drews, U., Wallwiener, D. Anatomical conditions for pelvic floor reconstruction with polypropylene implant and its application for the treatment of vaginal prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 131, 214-225 (2007).
- Carey, M., Slack, M., Higgs, P., Wynn-Williams, M., Cornish, A. Vaginal surgery for pelvic organ prolapse using mesh and a vaginal support device. BJOG An Int. J. Obstet. Gynaecol. 115 (3), 391-397 (2008).
- Urbankova, I., et al. Comparative anatomy of the ovine and female pelvis. Gynecol. Obstet. Invest. , (2016).
- Maurer, M. M., Röhrnbauer, B., Feola, a., Deprest, J., Mazza, E. Mechanical biocompatibility of prosthetic meshes: A comprehensive protocol for mechanical characterization. J. Mech. Behav. Biomed. Mater. 40, 42-58 (2014).
- Leval, J. Novel Surgical Technique for the Treatment of Female Stress Urinary Incontinence. Transobturator Vaginal Tape Inside-Out. Eur. Urol. 44 (6), 724-730 (2003).
- Reisenauer, C., Kirschniak, A., Drews, U., Wallwiener, D. Transobturator vaginal tape inside-out. Eur. J. Obstet. Gynecol. Reprod. Biol. 127 (1), 123-129 (2006).
- Bafghi, A., et al. Bowel perforation as late complication of tension-free vaginal tape. J Gynecol Obs. Biol Reprod. 34 (6), 606-607 (2005).
- Hinoul, P., Vanormelingen, L., Roovers, J. P., de Jonge, E., Smajda, S. Anatomical variability in the trajectory of the inside-out transobturator vaginal tape technique (TVT-O). Int. Urogynecol. J. Pelvic Floor Dysfunct. 18 (10), 1201-1206 (2007).
- Schaller, O., et al. . Illustrated Veterinary Anatomical Nomenclature. , (2007).
- . Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse. FDA Safety Communication. , (2016).
- Reinier, M., Groep, G. Final Opinion on the use of meshes in urogynecological surgery. SCENIHR- European Commission. , (2016).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone