Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
The 4-vessel Sampling Approach to Integrative Studies of Human Placental Physiology In Vivo
W tym Artykule
Podsumowanie
We present a detailed method to study human placental physiology in vivo at term. The method combines blood sampling from the incoming and outgoing vessels on the maternal and fetal sides of the placenta with ultrasound measurements of volume blood flow and placental tissue sampling.
Streszczenie
The human placenta is highly inaccessible for research while still in utero. The current understanding of human placental physiology in vivo is therefore largely based on animal studies, despite the high diversity among species in placental anatomy, hemodynamics and duration of the pregnancy. The vast majority of human placenta studies are ex vivo perfusion studies or in vitro trophoblast studies. Although in vitro studies and animal models are essential, extrapolation of the results from such studies to the human placenta in vivo is uncertain. We aimed to study human placenta physiology in vivo at term, and present a detailed protocol of the method. Exploiting the intraabdominal access to the uterine vein just before the uterine incision during planned cesarean section, we collect blood samples from the incoming and outgoing vessels on the maternal and fetal sides of the placenta. When combining concentration measurements from blood samples with volume blood flow measurements, we are able to quantify placental and fetal uptake and release of any compound. Furthermore, placental tissue samples from the same mother-fetus pairs can provide measurements of transporter density and activity and other aspects of placental functions in vivo. Through this integrative use of the 4-vessel sampling method we are able to test some of the current concepts of placental nutrient transfer and metabolism in vivo, both in normal and pathological pregnancies. Furthermore, this method enables the identification of substances secreted by the placenta to the maternal circulation, which could be an important contribution to the search for biomarkers of placenta dysfunction.
Wprowadzenie
According to the National Institutes of Health, USA, the placenta is the least understood organ in the human body 1,2,3. It is difficult to access and study the human placenta in vivo without imposing unethical risks on the ongoing pregnancy. Studies of placental function in the human are therefore largely based on in vitro and ex vivo models. The majority of previous in vivo studies of placental transport and metabolism have been performed in animals 4,5,6. However, as placental structure and functions vary considerably between species, extrapolation of results from animals to humans must be done with caution. Only a few smaller human in vivo studies have investigated placental and fetal uptake and transport under normal physiological conditions, and none have explored the integrated transfer of several compounds 7,8,9,10,11,12,13. These fundamental studies illustrate that in vivo studies of the human placenta are feasible, and that they may serve several purposes. First, current concepts of placental functions mainly derived from in vitro, ex vivo and animal studies may be tested in a human setting and thus provide novel and more specific insight into the human placenta. Second, properties of the dysfunctional placenta associated with aberrant fetal growth, preeclampsia, maternal diabetes, metabolic syndrome and other maternal metabolic disturbances may be better characterized. Third, human in vivo studies provide an opportunity to develop diagnostic and predictive tools of placental function.
On this background we aimed to establish a comprehensive collection of physiological data to investigate human placental function in vivo. During a planned cesarean section, we exploit the intraabdominal access to the uterine vein to collect blood samples from the incoming and outgoing vessels on the maternal and fetal sides of the placenta (the 4-vessel sampling method). These samples are used to calculate the paired arteriovenous concentration differences of nutrients and other substances 14. In addition, we measure volume blood flow on both sides of the placenta by ultrasound. Consequently, placental and fetal uptake of any compound may be quantified. Further, it is possible to determine substances released by the placenta to the maternal and fetal circulations 15,16,17. When combined with clinical parameters of mother and child, and analyses of placental and other relevant tissues, this method has the exciting potential to integrate many aspects of placental functions in vivo in the same mother-fetus pairs.
Protokół
The study was approved by the data protection officials at Oslo University Hospital and the Regional Committee for Medical and Health Research Ethics, Southern Norway 2419/2011. All participants signed a written informed consent at inclusion.
1. Preparations
NOTE: A timeline for the procedures is outlined in Figure 1.
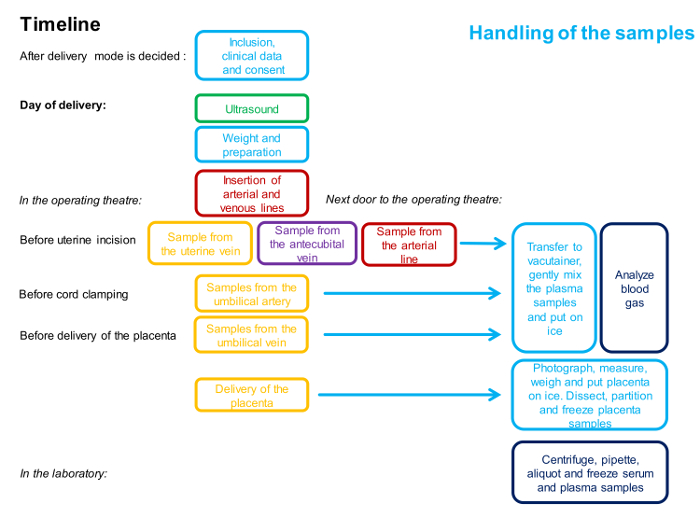
Figure 1: Flowchart Describing the Timing and the Personnel Involved in the 4-vessel Sampling Procedure.
One color represents one person. Detailed description of the method is given in the protocol. Please click here to view a larger version of this figure.
- Staff
- Make sure all required personnel are available: a highly skilled Fetal Medicine specialist conducting the ultrasound measurements, two obstetricians conducting the surgery, one of the obstetricians and two nurses collecting the samples, one assistant handling the blood gas analyses and one assistant handling the other samples consecutively and immediately following the collection.
NOTE: In cases of more advanced collection of placental tissue, an additional person is required.
- Make sure all required personnel are available: a highly skilled Fetal Medicine specialist conducting the ultrasound measurements, two obstetricians conducting the surgery, one of the obstetricians and two nurses collecting the samples, one assistant handling the blood gas analyses and one assistant handling the other samples consecutively and immediately following the collection.
- Equipment
- Prepare the equipment, 50 mL of ice cold 1 M phosphate buffered saline (PBS), 25 mL of cold RNA stabilizing solution and 5 x 0.5 mL of optimal cutting temperature compound (OCT). Label the vacutainers and tubes. See tentative list of equipment.
2. Maternal Characteristics
- Record the maternal clinical and non-clinical characteristics at inclusion and repeat relevant questions and measurements, including weight, at the time of delivery. Record the duration of the fasting period prior to the cesarean section, and any hypotensive episodes occurring during the surgery.
Note: Include the minimal maternal clinical dataset reported in a recent publication from Global Pregnancy CoLaboratory (COLAB). This article also includes some very important aspects in choosing study populations and should be addressed while planning the study 18. - Consider recording paternal characteristics, including ethnicity, age and body mass index (BMI).
3. Ultrasound
- Perform the Doppler ultrasound examination on the day of the delivery, with the women in a fasting state. Perform the examination during a period of fetal quiescence, with the woman in semi-supine position, tilted slightly laterally opposite to the region of interest in order to avoid compression of the aorta and vena cava. Monitor the output intensity by the mechanical and thermal indices on the display.
- Umbilical vein
- Visualize the umbilical vein in a sagittal or oblique transection of the fetal abdomen. Measure the internal vessel diameter in the straight portion of the intra-abdominal umbilical vein, before any visible branches. Use regular B-mode and visualize the vessel in a perpendicular insonation angle for diameter measurements and keep several optimal frames for later measurements to minimize the effect of pulsatile diameter changes.
- Repeat the measurements five to ten times 19.
- At the same site, use Doppler ultrasound and adjust the probe to get an insonation angle as low as possible (always <30°) in order to measure the time-averaged maximum velocity (TAMX). Obtain the velocity over a period of 3 - 5 s (non-pulsating flow).
- Visualize the umbilical vein in a sagittal or oblique transection of the fetal abdomen. Measure the internal vessel diameter in the straight portion of the intra-abdominal umbilical vein, before any visible branches. Use regular B-mode and visualize the vessel in a perpendicular insonation angle for diameter measurements and keep several optimal frames for later measurements to minimize the effect of pulsatile diameter changes.
- Uterine artery
- Use Doppler ultrasound to visualize the uterine artery as it crosses the external iliac artery, immediately after it branches from the internal iliac artery. Adjust the probe at this site to get a low insonation angle (always <30°) and measure TAMX. Obtain the velocity as the mean velocity of three heart cycles.
- As it is unlikely to get a perpendicular angle at the same site as TAMX is measured, follow the vessel distally to get a correct angle for diameter measurements as close to the sites of diameter measurements as achievable. Exclude the diameter measurements if any visible vessels branch off before this site as evaluated by color Doppler ultrasound.
- Use regular B-mode and visualize the vessel in a perpendicular insonation angle for diameter measurements and keep several optimal frames for later measurements to minimize the effect of pulsatile diameter changes.
- Repeat the measurements five to ten times 19.
- Note the position of the placenta.
4. 4-vessel Blood Sampling
NOTE: The timeline for the procedures is outlined in Figure 1 and an overview of the samples is illustrated in Figure 2.
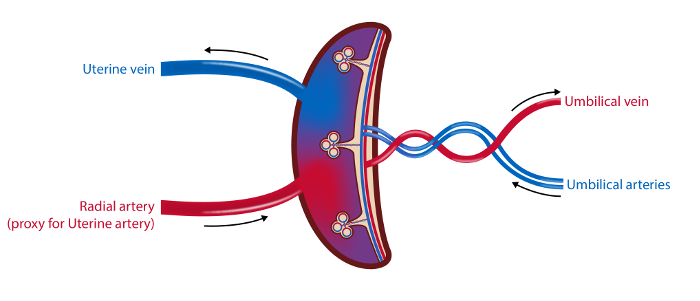
Figure 2: Schematic Illustration of the Placental Vasculature and the Sampling Sites.
In the 4-vessel sampling method blood samples are drawn from the uterine vein, the radial artery (as a proxy for the uterine artery) and the umbilical arteries and vein. Blood flow in the uterine artery and the umbilical vein is measured by ultrasound. Tissue samples from the placenta are collected. Illustration: Øystein H. Horgmo, University of Oslo. Please click here to view a larger version of this figure.
- Safety procedures
- Provide all personnel in the operation theater with gloves, surgical scrub suits, masks and headwear.
- Provide the surgeons and research personnel in contact with the operation field with surgical scrub suits, masks, headwear, gowns and double gloves. Glasses are optional.
- Provide personnel handling the blood samples with gloves.
- Provide personnel handling the placenta samples with gloves and surgical mask. Homogenization requires the use of hoods.
- Preparation in the operation theater
- Give a briefing and hand the equipment to all personnel that will assist the sampling before onset of surgery.
- Address the anesthesiologist and anesthesiology nurse who will assist with the necessary peripheral arterial and venous access, and ensure that no liquids are given intravenously before sampling.
- Give three syringes (10 mL) without needles to the person assisting with the antecubital vein sample and two syringes (one 20 mL and one 10 mL) and one blood gas syringe (with heparin) to the person assisting with the radial artery.
- Prepare two sterile syringes (20 mL), five sterile syringes (10 mL), three "butterfly needles" and two blood gas syringes for the operation field.
- Access to blood vessels.
- Follow standard procedure before the cesarean section to assure peripheral intravenous (iv) access.
NOTE: The antecubital vein is preferable because it is easier to draw samples from this site. - Localize the radial artery at the wrist by ultrasound or by palpation. Following 0.5 mL of subcutaneous lidocaine analgesia, place an arterial line into the radial artery. Abandon the sampling from this site in case of three failed insertions, or if the woman experiences pain during the insertion.
NOTE: Perform the surgical procedure of cesarean section according to standard procedure. Only the adjustments needed for the sampling procedure are underlined below.
- Follow standard procedure before the cesarean section to assure peripheral intravenous (iv) access.
- Maternal blood samples
NOTE: Obtain all three maternal blood samples (uterine vein, radial artery and antecubital vein) simultaneously before the uterine incision.- For the uterine vein, after opening the abdominal cavity, use a retractor to lift the abdominal wall and expose the main branches of the uterine veins on the anterolateral sides of the uterus. Obtain blood from uterine vein branches at the same side as the placenta whenever possible or use the most prominent vein plexus if the placenta is located in the uterine midline.
- Insert a butterfly needle on a blood gas syringe in the uterine vein at an angle of approximately 30 degrees and collect blood through gentle aspiration to avoid hemolysis. While carefully securing the iv position of the butterfly needle, replace the filled blood gas syringe by a 20 mL and a 10 mL syringe consecutively.
NOTE: Optimal access is best ensured when standing on the contralateral side of the chosen uterine vein.
- Insert a butterfly needle on a blood gas syringe in the uterine vein at an angle of approximately 30 degrees and collect blood through gentle aspiration to avoid hemolysis. While carefully securing the iv position of the butterfly needle, replace the filled blood gas syringe by a 20 mL and a 10 mL syringe consecutively.
- For the radial artery, aspirate from the intra-arterial line. Discard the first 5 mL, and then aspirate 3 mL in heparin syringe for blood gas analyses, followed by 3 mL in two syringes (20 + 10 mL).
- For the antecubital vein, aspirate gently from the intravenous catheter. Discard the first 5 mL, and then aspirate 30 mL in three syringes (10 mL).
- Perform a final inspection of the sampling site on the uterine vein before starting to close the abdomen.
- For the uterine vein, after opening the abdominal cavity, use a retractor to lift the abdominal wall and expose the main branches of the uterine veins on the anterolateral sides of the uterus. Obtain blood from uterine vein branches at the same side as the placenta whenever possible or use the most prominent vein plexus if the placenta is located in the uterine midline.
- Fetal blood samples
- When the child is born, immediately aspirate blood from the umbilical artery, without clamping the umbilical cord or delivering the placenta. Start with the syringe for blood gas analysis, and follow with three 10 mL syringes if possible.
- When the arterial samples are secured, clamp the cord and hand the child to the midwife before sampling from the umbilical vein (blood gas and 20 + 10 mL syringes).
NOTE: Obtain all umbilical samples within seconds of delivery and with the placenta in situ unless it has detached spontaneously. - Follow the Norwegian recommendations on late cord clamping. In case of a distressed child, clamp and cut the cord immediately and the hand the child to the midwife and neonatologist.
- Handling of blood samples
- Put the blood gas syringes on ice while preparing the rest of the blood samples, and analyze them in a blood gas analyzer within 5 min.
- Transfer the blood samples immediately to vacutainers and place the plasma tubes on a rocker for 1 - 2 min before putting them on ice. Leave the serum tubes on the laboratory bench to settle for 30 minutes.
NOTE: This is a critical step in the procedure that needs extra attention because samples from all five sites have to be prepared simultaneously to ensure good quality. - Centrifuge the plasma samples as soon as possible, and within 30 min, at 6 °C, 2,500 x g for 20 min.
- After 30 min, centrifuge the serum samples at room temperature for 10 min at 2,500 x g.
- Aliquot the supernatants carefully to 2 mL cryo tubes, leaving 0.5 mL of the supernatant above the pellet to ensure platelet free plasma.
- Store the samples at -80 °C.
5. Collection of Placental Tissue
- Place the placenta flat down on an ice chilled dissection tray as soon as possible after it has been delivered. Photographand measure the longest diameter and the diameter at 90 degrees.
- Weigh the placenta.
- Record the weight, the two diameters, any gross pathology, number of vessels in the cord and the time interval from delivery to when the placenta was placed on ice.
NOTE: Send the placenta to pathological examination if clinically indicated. - Place the placenta with the maternal surface facing up and identify 4 - 5 sampling sites randomly located in each quadrant of the placenta, avoiding areas of frank pathology. Remove the decidua using scissors to cut away 3 - 5 mm from the maternal surface. Collect a 1 - 2 cm3 piece of villous tissue from each site.
- Wash the collected tissue gently in 50 mL of cold 1M PBS. Divide into several pieces from each sampling site and aliquot.
Note: The size of the placenta pieces will depend on the planned analyzes. - Add aliquots of 0.1 - 0.5 cm3 tissue samples to 5 cryo tubes and snap freeze in liquid nitrogen.
- Add small pieces of 0.1 - 0.2 cm3 to the tube with 25 mL of RNA stabilization solution. Store at 4 °C for 24 h, discard the RNA stabilization solution and replace it. Freeze.
- Add pieces of 0.5 cm3 to the 5 cryo tubes with 0.5 mL of OCT, top up with OCT, mix and freeze.
- Store the samples at -80 °C until analysis.
NOTE: Burton et al. provides an excellent overview of practical aspects of placental sampling depending on the analyses planned. 20 Consider to prepare the remaining tissue for isolation of the microvillous and basal membranes, and to collect decidual tissue by vacuum suction technique. 21,22
6. Neonatal Characteristics
- Record the neonatal characteristics, including Apgar-score (1, 5 and 10 min), sex, weight, length, gestational age and admission to Newborn Intensive Care Unit (length and outcome of stay).
- Consider measuring neonatal body composition by anthropometric measurements, air-displacement plethysmograph or dual X-ray absorptiometry.23,24
7. Calculations
- Assume similar blood composition in the radial and uterine artery and calculate the uteroplacental arteriovenous concentration difference.
Uteroplacental arteriovenous concentration difference = CA - CV
Umbilical venous - arterial concentration difference = Cv - Ca
Where C is concentration with subscripts: A, the radial artery; V, the uterine vein; v the umbilical vein and a, the umbilical artery. - Calculate the volume blood flow, mL/min (Q):
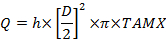
Where D is the vessel diameter (cm), TAMX is time averaged maximum velocity and h is the coefficient for the spatial blood velocity profile. Use 0.5 as the coefficient for the umbilical vein and 0.6 for the uterine artery25,26. - Calculate the placental uptake and release according to Fick's principle:
Uteroplacental uptake = (CA - CV) x Qm
Fetal uptake = (Cv - Ca) x Qf
Subscripts: m, maternal and f, fetal.
Wyniki
The 4-vessel sampling method is applicable in clinical practice and we have successfully obtained blood samples from 209 mother/infant-pairs. In 128 of these we also achieved to measure volume blood flow. Complete 4-vessel sampling and good quality flow measurements of both maternal and fetal vessels were obtained in 70 mother-fetus pairs (Figure 3). In addition, we have so far collected blood and placenta samples from 30 preeclamptic patients. We have previo...
Dyskusje
The placenta 4-vessel sampling method is relevant for three main purposes. First, it can be used to study how specific substances are taken up by the placenta on the maternal side and possibly transferred to the umbilical circulation and the fetus, as demonstrated by our glucose and amino acid studies. Second, the method is highly relevant to study substances produced by the placenta and released to the maternal or the fetal circulations, as demonstrated by the progesterone results. Third, it may be useful to study how t...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
First and foremost, we sincerely thank the mothers who participated in this project. Next, we acknowledge all the personnel that assisted and facilitated the sampling procedure, the Anesthesiologist, the nurse anesthesiologist and the surgical nurses. The project would not have been possible without funding from the South-Eastern Norway Regional Health Authority and Norwegian Advisory Unit on Women's Health, Oslo University and local funding provided by Oslo University Hospital.
Materiały
| Name | Company | Catalog Number | Comments |
| Maternal body composition | |||
| Impedance scale | Tanita | or similar | |
| Ultrasound measurements | |||
| Sequoia 512 ultrasound machine | Acuson | equipped with a curved transducer with colour and pulsewave Doppler (frequency bandwidth 2-6 MHz) | |
| Blood samples | |||
| Arerial cannula | BD Medical | 682245 | or similar |
| 20cc Eccentric Luer Tip Syringe without Needle | Termo | SS-20ES | or similar. 3 needed. |
| 10cc Eccentric Luer Tip Syringe without Needle | Termo | SS-10ES | or similar. 9 needed. |
| 5cc 6% Luer Syringe without Needle | Termo | SS-05S1 | or similar. 2 needed. |
| Arterial blood gas syringe | Radiometer Medical | or similar. 4 needed. | |
| Sterile winged needle connected to flexible tubing, 21 gauge | Greiner Bio-One | 450081 | (intended for single use).3 needed. |
| Vacutainer tube 6 mL EDTA | Greiner Bio-One | 456043 | or similar. Label with sample site. 10 needed. |
| Vacutainer tube 5 ml LH Lithium Heparin Separator | Greiner Bio-One | 456305 | or similar. Label with sample site. 5 needed. |
| Vacutainer tube 6 mL Serum Clot Activator | Greiner Bio-One | 456089 | or similar. Label with sample site. 5 needed. |
| Vacutainer tube 3 ml 9NC Coagulation sodium citrate 3,2% | Greiner Bio-One | 454334 | or similar. Label with sample site. 5 needed. |
| Cryogenic vials, 2.0 mL | Corning | 430488 | or similar. Label with sample site, serum/type of plasma and ID. 90 needed. |
| Marked trays to transport the syringes | to transport the blood samples in the operation theatre | ||
| Rocker | for gentle mixing of the samples | ||
| Ice | in styrofoam box | ||
| Liquid nitrogen | in appropriate container | ||
| Placenta samples | |||
| Metal tray | |||
| Ice | in styrofoam box | ||
| Calibrated scale | |||
| Metal ruler | |||
| 1 M Phosphate buffered saline | Sigma | D1408 | or similar. Dilute 10 M to 1M before use |
| RNA stabilization solution | Sigma | R0901-500ML | or similar |
| Optimal Cutting Temperature (O.C.T.) compound | vwr | 361603E | or similar |
| Cryogenic vials, 2.0 mL | Corning | 430488 | or similar. Label with sample site. content and ID. 10 needed. |
| Centrifuge tubes, conical bottom 50 mL | Greiner Bio-One | 227,285 | or similar. Label with "RNA later", sample site and ID. 2 needed. |
| Liquid nitrogen | in appropriate container | ||
| Fetal body composition | |||
| Calibrated scale | |||
| Measuring tape |
Odniesienia
- Jansson, T., Powell, T. L. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond). 113 (1), 1-13 (2007).
- Hanson, M. A., Gluckman, P. D. Early developmental conditioning of later health and disease: physiology or pathophysiology. Physiol Rev. 94 (4), 1027-1076 (2014).
- Guttmacher, A. E., Spong, C. Y. The human placenta project: it's time for real time. Am J Obstet Gynecol. 213, 3-5 (2015).
- Battaglia, F. C., Regnault, T. R. Placental transport and metabolism of amino acids. Placenta. 22 (2-3), 145-161 (2001).
- Hay, W. W. Placental-fetal glucose exchange and fetal glucose metabolism. Trans Am Clin Climatol Assoc. 117, 321-339 (2006).
- Woollett, L. A. Review: Transport of maternal cholesterol to the fetal circulation. Placenta. 32, 218-221 (2011).
- Prenton, M. A., Young, M. Umbilical vein-artery and uterine arterio-venous plasma amino acid differences (in the human subject). J Obstet Gynaecol Br Commonw. 76 (5), 404-411 (1969).
- Cetin, I., et al. Plasma and erythrocyte amino acids in mother and fetus. Biol Neonate. 60 (2), 83-91 (1991).
- Filshie, G. M., Anstey, M. D. The distribution of arachidonic acid in plasma and tissues of patients near term undergoing elective or emergency Caesarean section. Br J Obstet Gynaecol. 85 (2), 119-123 (1978).
- Haberey, P. P., Schaefer, A., Nisand, I., Dellenbach, P. The fate and importance of fetal lactate in the human placenta -a new hypothesis. J Perinat Med. 10 (2), 127-129 (1982).
- Prendergast, C. H., et al. Glucose production by the human placenta in vivo. Placenta. 20 (7), 591-598 (1999).
- Metzger, B. E., Rodeck, C., Freinkel, N., Price, J., Young, M. Transplacental arteriovenous gradients for glucose, insulin, glucagon and placental lactogen during normoglycaemia in human pregnancy at term. Placenta. 6 (4), 347-354 (1985).
- Zamudio, S., et al. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One. 5 (1), 8551 (2010).
- Holme, A. M., Roland, M. C., Lorentzen, B., Michelsen, T. M., Henriksen, T. Placental glucose transfer: a human in vivo study. PLoS One. 10 (2), 0117084 (2015).
- Holme, A. M., Roland, M. C., Henriksen, T., Michelsen, T. M. In vivo uteroplacental release of placental growth factor and soluble Fms-like tyrosine kinase-1 in normal and preeclamptic pregnancies. Am J Obstet Gynecol. 215 (6), 781-782 (2016).
- Paasche Roland, M. C., Lorentzen, B., Godang, K., Henriksen, T. Uteroplacental arterio-venous difference in soluble VEGFR-1 (sFlt-1), but not in soluble endoglin concentrations in preeclampsia. Placenta. 33 (3), 224-226 (2012).
- Brar, H. S., et al. Uteroplacental unit as a source of elevated circulating prorenin levels in normal pregnancy. Am J Obstet Gynecol. 155 (6), 1223-1226 (1986).
- Myatt, L., et al. Strategy for standardization of preeclampsia research study design. Hypertension. 63 (6), 1293-1301 (2014).
- Kiserud, T., Rasmussen, S. How repeat measurements affect the mean diameter of the umbilical vein and the ductus venosus. Ultrasound Obstet Gynecol. 11 (6), 419-425 (1998).
- Burton, G. J., et al. Optimising sample collection for placental research. Placenta. 35 (1), 9-22 (2014).
- Illsley, N. P., Wang, Z. Q., Gray, A., Sellers, M. C., Jacobs, M. M. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta. 1029 (2), 218-226 (1990).
- Staff, A. C., Ranheim, T., Khoury, J., Henriksen, T. Increased contents of phospholipids, cholesterol, and lipid peroxides in decidua basalis in women with preeclampsia. Am J Obstet Gynecol. 180 (3), 587-592 (1999).
- Catalano, P. M., Thomas, A. J., Avallone, D. A., Amini, S. B. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. 173 (4), 1176-1181 (1995).
- Ellis, K. J., et al. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 85 (1), 90-95 (2007).
- Haugen, G., Kiserud, T., Godfrey, K., Crozier, S., Hanson, M. Portal and umbilical venous blood supply to the liver in the human fetus near term. Ultrasound Obstet Gynecol. 24 (6), 599-605 (2004).
- Acharya, G., et al. Experimental validation of uterine artery volume blood flow measurement by Doppler ultrasonography in pregnant sheep. Ultrasound Obstet Gynecol. 29 (4), 401-406 (2007).
- Wu, X., et al. Glutamate-glutamine cycle and exchange in the placenta-fetus unit during late pregnancy. Amino Acids. 47 (1), 45-53 (2015).
- Tuckey, R. C. Progesterone synthesis by the human placenta. Placenta. 26 (4), 273-281 (2005).
- Simmons, M. A., Meschia, G., Makowski, E. L., Battaglia, F. C. Fetal metabolic response to maternal starvation. Pediatr Res. 8 (10), 830-836 (1974).
- Simmons, M. A., Jones, M. D., Battaglia, F. C., Meschia, G. Insulin effect on fetal glucose utilization. Pediatr Res. 12 (2), 90-92 (1978).
- Bujold, E., et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 18 (1), 9-16 (2005).
- Jansson, T., Aye, I. L., Goberdhan, D. C. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta. 33, 23-29 (2012).
- Cetin, I. Placental transport of amino acids in normal and growth-restricted pregnancies. Eur J Obstet Gynecol Reprod Biol. 110, 50-54 (2003).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone