Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Chemical Precipitation Method for the Synthesis of Nb2O5 Modified Bulk Nickel Catalysts with High Specific Surface Area
W tym Artykule
Podsumowanie
A protocol for the synthesis of sponge-like and fold-like Ni1-xNbxO nanoparticles by chemical precipitation is presented.
Streszczenie
We demonstrate a method for the synthesis of NixNb1-xO catalysts with sponge-like and fold-like nanostructures. By varying the Nb:Ni ratio, a series of NixNb1-xO nanoparticles with different atomic compositions (x = 0.03, 0.08, 0.15, and 0.20) have been prepared by chemical precipitation. These NixNb1-xO catalysts are characterized by X-ray diffraction, X-ray photoelectron spectroscopy, and scanning electron microscopy. The study revealed the sponge-like and fold-like appearance of Ni0.97Nb0.03O and Ni0.92Nb0.08O on the NiO surface, and the larger surface area of these NixNb1-xO catalysts, compared with the bulk NiO. Maximum surface area of 173 m2/g can be obtained for Ni0.92Nb0.08O catalysts. In addition, the catalytic hydroconversion of lignin-derived compounds using the synthesized Ni0.92Nb0.08O catalysts have been investigated.
Wprowadzenie
The preparation of nanocomposites has received increasing attention due to their crucial application in various field. To prepare Ni-Nb-O mixed oxide nanoparticles,1,2,3,4,5,6 different methods have been developed such as dry mixing method,7,8 evaporation method,9,10,11,12,13 sol gel method,14 thermal decomposition method,15 and auto-combustion.16 In a typical evaporation method9, aqueous solutions containing the appropriate amount of metal precursors, nickel nitrate hexahydrate and ammonium niobium oxalate were heated at 70 °C. After the removal of solvent and further drying and calcination, the mixed oxide was obtained. These oxide catalysts exhibit excellent catalytic activity and selectivity towards the oxidative dehydrogenation (ODH) of ethane, which is related to the electronic and structural rearrangement induced by the incorporation of niobium cations in the NiO lattice.11 The insertion of Nb drastically decreases the electrophilic oxygen species, which is responsible for the oxidation reactions of ethane12. As a result, extensions of this method have been done on the preparation of different types of mixed Ni-Me-O oxides, where Me = Li, Mg, Al, Ga, Ti and Ta.13 It is found that the variation of metal dopants could alter the unselective and electrophilic oxygen radicals of NiO, thus systematically tune the ODH activity and selectivity towards ethane. However, generally the surface area of these oxides is relatively small (< 100 m2/g), due to the extended phase segregation and formation of large Nb2O5 crystallites, and thus hampered their uses in other catalytic applications.
Dry mixing method, also known as the solid-state grinding method, is another commonly used method to prepare the mixed-oxide catalysts. Since the catalytic materials are obtained in a solvent-free way, this method provides a promising green and sustainable alternative to the preparation of mixed-oxide. The highest surface area obtained by this method is 172 m2/g for Ni80Nb20 at calcination temperature of 250 °C.8 However, this solid-state method is not reliable as reactants are not well mixed on the atomic scale. Therefore, for better control of chemical homogeneity and specific particle size distribution and morphology, other suitable methods to prepare Ni-Nb-O mixed oxide nanoparticles are still being sought.7
Among various strategies in the development of nanoparticles, chemical precipitation serves as one of the promising methods to develop the nanocatalysts, since it allows the complete precipitation of the metal ions. Also, nanoparticles of higher surface areas are commonly prepared by using this method. To improve the catalytic properties of Ni-Nb-O nanoparticles, we herein report the protocol for the synthesis of a series of Ni-Nb-O mixed oxide catalysts with high surface area by chemical precipitation method. We demonstrated that the Nb:Ni molar ratio is a crucial factor in determining the catalytic activity of the oxides towards the hydrodeoxygenation of lignin-derived organic compounds. With high Nb:Ni ratio above 0.087, inactive NiNb2O6 species were formed. Ni0.92Nb0.08O, which had the largest surface area (173 m2/g), exhibits fold-like nanosheets structures and showed the best activity and selectivity towards the hydrodeoxygenation of anisole to cyclohexane.
Access restricted. Please log in or start a trial to view this content.
Protokół
Caution: For the proper handling methods, properties and toxicities of the chemicals described in this paper, refer to the relevant material safety data sheets (MSDS). Some of the chemicals used are toxic and carcinogenic and special cares must be taken. Nanomaterials may potentially pose safety hazards and health effects. Inhalation and skin contact should be avoided. Safety precaution must be exercised, such as performing the catalyst synthesis in the fume hood and catalyst performance evaluation with autoclave reactors. Personal protective equipment must be worn.
1. Preparation of Ni0.97Nb0.03O Catalysts where Nb:(Ni+Nb) molar ratios equal to 0.03
- Combine 0.161 g of niobium (V) oxalate hydrate with 2.821 g of nickel nitrate in 100 mL of deionized water in a 250-mL three-necked round bottom flask equipped with a stir bar.
- Stir the solution at 50 rpm and 70 °C to dissolve the compounds until the disappearance of precipitate using a heating magnetic stirrer.
- Raise the temperature rapidly to 80 °C at a rate of 2 °C/min.
- Add a mixed basic solution [aqueous ammonium hydroxide (50 mL, 1.0 M) and sodium hydroxide (50 mL, 0.2 M)] into the reaction mixture dropwise until the pH of the Ni/Nb solution reaches 9.0.
- While stirring the reaction mixture, raise the temperature to 120 °C at 2 °C/min.
- Stir the reaction mixture overnight at 50 rpm at 120 °C until the complete disappearance of the green color of the solution.
- Perform inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis for the solution to evaluate the concentration of remaining Ni2+ and Nb5+ ions in the solution and ensure the complete precipitation of remaining nickel nitrate.
- Collect the solid by filtration using Büchner flask. Wash the solid by adding 2 L deionized water repeatedly within 20 min to remove the residual Na+ cation.
- Collect the solid in a watch glass. Dry the solid at 110 °C for 12 h in dry oven.
- Calcine by heating the solids in synthetic air (20 mL/min O2 and 80 mL/min N2) at 450 °C for 5 h in tube furnace. Check all glassware for defect prior to use the high temperature of reaction.
- After the calcination, obtain 1 g of Ni0.97Nb0.03O catalyst. Use appropriate protective equipment such as safety glasses, gloves, lab coat, and fume hood to perform the nanocrystal reaction due to potential safety hazards and health effects of the nanomaterials.
2. Preparation of Ni0.92Nb0.08O Catalysts where Nb:(Ni+Nb) molar ratios equal to 0.08
- This procedure is similar to that of 1 except for the first two steps:
- Dissolve 0.43 g of niobium (V) oxalate hydrate in 100 mL of deionized water.
- Separately, dissolve 2.675 g of nickel nitrate in 100 mL of deionized water.
3. Preparation of Ni0.85Nb0.15O Catalysts where Nb:(Ni+Nb) molar ratios equal to 0.15
- The procedure is similar to that of 1 except for the first two steps:
- Dissolve 0.807 g of niobium (V) oxalate hydrate in 100 mL of deionized water.
- Separately, dissolve 2.472 g of nickel nitrate in 100 mL of deionized water.
4. Preparation of Ni0.80Nb0.20O Catalysts where Nb:(Ni+Nb) molar ratios equal to 0.20
- The procedure is similar to that of 1 except for the first two steps:
- Dissolve 1.076 g of niobium (V) oxalate hydrate in 100 mL of deionized water.
- Separately, dissolve 2.326 g of nickel nitrate in 100 mL of deionized water.
5. Preparation of Nb2O5 using chemical precipitation method
- Calcine niobic acid (Nb2O5·nH2O) in synthetic air for 5 h at 450 °C to obtain pure Nb2O5 particles.
NOTE: Confirm the completion of reaction using X-ray powder diffraction (XRD) analysis, where Nb2O5·nH2O is Amorphous and Nb2O5 is crystalline. According to the analysis, the calcination for 5 h at 450 °C was enough to complete the reaction.
6. Synthesis of β-O-4 lignin model compound, 2-(2-methoxyphenoxy)-1-phenylethan-1-one
- Dissolve bromoacetophenone (9.0 g, 45 mmol) and 2-methoxyphenol (6.6 g, 53 mmol) in 200 mL of dimethylformamide (DMF) in a 500-mL conical flask with a magnetic stirrer. Use appropriate protective equipment and fume hood to perform the reaction using corrosive and carcinogenic chemicals and reagents.
- Mix the above DMF solution with potassium hydroxide (3.0 g, 53 mmol) and stir the mixture overnight at 50 rpm at room temperature using magnetic stirrers.
- Extract the product with the mixture solution of 200 mL of H2O and 600 mL of diethyl ether (1:3, v/v) using separation funnel. Obtain the upper diethyl ether layer of the solution.
- Add MgSO4 (10 g) to absorb moisture of the diethyl ether solution. Filter the MgSO4 to obtain the diethyl ether solution by using filter paper and funnel.
- After removal of the diethyl ether solution under reduced pressure at 0.08 MPa using rotary evaporator, dissolve the residue in 5 mL of ethanol.
- Slowly evaporate the ethanol solvent to recrystallize the product in a 10-mL beaker. Obtain the product (11.5 g) as yellowish powder and the yield of product is 90% based on bromoacetophenone. From the 1H NMR analysis, 1H NMR (DMSO): δ 3.78 (s, 3H, OCH3), 5.54 (s, 2H, CH2), 6.82-8.01 (m, 9H, aromatic) ppm.17
7. Hydrodeoxygenation of Lignin-derived Aromatic Ether
NOTE: The chosen lignin-derived aromatic ether is anisole in this experiment and the catalyst is Ni0.92Nb0.08O. Use appropriate protective equipment and fume hood to perform the reaction using carcinogenic reagents.
- Equip a 50-mL stainless steel autoclave reactor with a heater and a magnetic stirrer.
- Reduce the Ni0.92Nb0.08O catalyst (1 g) obtained from step 2 in the autoclave reactor in H2 atmosphere at 400 °C for 2 h, and then passivate the catalyst under Argon (50 mL/min) overnight.
- Dissolve anisole (1.1712 g, 8 wt%) into n-decane (20 mL) with the use of n-dodecane (0.2928 g, 2 wt%) as an internal standard for quantitative gas chromatography (GC) analysis.
- Introduce the reduced catalysts (0.1 g) in into the autoclave reactor rapidly to avoid long exposure time with air (< 5 mins).
- Seal the autoclave reactor, purge with H2 repeatedly (3 times, at 3 MPa pressure) to eliminate air, and then the reaction mixture at atmosphere pressure.
- Set the stirring speed at 700 rpm.
- After heating to the desired temperature at 160-210 °C at 2 °C/min, pressurize the autoclave reactor to 3 MPa and set the zero-time point (t = 0).
NOTE: The temperature range of 160-210 °C is appropriate in this report. - Subsequently, cool the mixture to room temperature at 10 °C/min immediately and analyze the deoxygenated products using gas chromatography with mass selective detector.17
- Determine the conversion of lignin model compound according to the following equation:

- Determine the product selectivity according to the following equation:
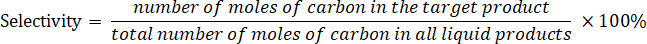
Access restricted. Please log in or start a trial to view this content.
Wyniki
X-ray diffraction (XRD) patterns (Figure 1 and Figure 2), BET surface areas, temperature-programmed reduction of hydrogen with hydrogen (H2-TPR), scanning electron microscopy (SEM) equipped with an energy-dispersive X-ray (EDX) analyzer, X-ray photoelectron spectroscopy (XPS) were collected for the nanoparticles NiO, Ni-Nb-O and Nb2O5 oxides17 (Figure 3...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
One of the common methods to prepare the nickel-doped bulk niobium oxide nanoparticles is rotary evaporation method.9 By employing various pressure and temperature conditions during the process of rotary evaporation, the precipitation of Ni-Nb-O particles commerce with the slow removal of the solvent. In contrast to the rotary evaporation method, the chemical precipitation method reported in this study has received increasing attention to prepare the nanoparticles as this do not require the remova...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
We have nothing to disclose.
Podziękowania
We gratefully acknowledge the financial support provided by National Key Research & Development Program of the Ministry of Science and Technology of China (2016YFB0600305), National Natural Science Foundation of China (Nos. 21573031 and 21373038), Program for Excellent Talents in Dalian City (2016RD09) and Technological and Higher Education Institute of Hong Kong (THEi SG1617105 and THEi SG1617127).
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Niobium(V) oxalate hydrate, 98% | Alfa | L04481902 | |
| Nickel nitrate hexahydrate, 99% | Aladdin | N108891 | |
| Sodium hydroxide, 98% | Aladdin | S111501 | |
| Ammonium hydroxide, 23-25% | Aladdin | A112077 | |
| Anisole, 99% | Sinopharm | 81001728 | |
| Diphenyl ether, 98% | Aladdin | D110644 | |
| Phenol, 98% | Sinopharm | 100153008 | |
| 2-Methoxyphenol, 98% | Sinopharm | 30114526 | |
| Vanillin, 99.5% | Sinopharm | 69024316 | |
| Potassium hydroxide, AR | Aladdin | P112284 | |
| N,N-Dimethylformamide, 99.5% | Sinopharm | 40016462 | |
| 2-Bromoacetophenone,98% | Aladdin | B103328 | |
| Diethyl ether,99.5% | Sinopharm | 10009318 | |
| Decane,98% | Aladdin | D105231 | |
| Dodecane,99% | Aladdin | D119697 | |
| Niobic acid | CBMM | 1313968 | |
| Heating and Drying Oven | DHG Series (shanghai jinghong laboratory instrument co. ltd) | ||
| Autoclave Reactor | CJF-0.05—0.1L (Dalian Tongda Equipment Technology Development Co., Ltd) | ||
| Tube furnace | SK2-1-10/12 (Luoyang Huaxulier Electric Stove Co., Ltd) | ||
| Heating magnetic stirrer | DF-101 (Yu Hua Instrument Co. Ltd.) | ||
| Rotary evaporator | RE-3000A (Shanghai Yarong Biochemical Instrument Factory) | ||
| Synthetic air | |||
| Hydrogen gas | |||
| Argon gas |
Odniesienia
- Zhou, Y., Yang, M., Sun, K., Tang, Z., Kotov, N. A. Similar topological origin of chiral centers in organic and nanoscale inorganic structures: effect of stabilizer chirality on optical isomerism and growth of CdTe nanocrystals. J. Am. Chem. Soc. 132 (17), 6006-6013 (2010).
- Zhou, Y., et al. Optical Coupling Between Chiral Biomolecules and Semiconductor Nanoparticles: Size-Dependent Circular Dichroism Absorption. Angew. Chem. Int. Ed. 50, 11456-11459 (2011).
- Li, Z., et al. Reversible plasmonic circular dichroism of Au nanorod and DNA assemblies. J. Am. Chem. Soc. 134 (7), 3322-3325 (2012).
- Zhu, Z., et al. Manipulation of collective optical activity in one-dimensional plasmonic assembly. ACS Nano. 6 (3), 2326-2332 (2012).
- Liu, W., et al. Gold nanorod@chiral mesoporous silica core-shell nanoparticles with unique optical properties. J. Am. Chem. Soc. 135 (26), 9659-9664 (2013).
- Han, B., Zhu, Z., Li, Z., Zhang, W., Tang, Z. Conformation Modulated Optical Activity Enhancement in Chiral Cysteine and Au Nanorod Assemblies. J. Am. Chem. Soc. 136, 16104-16107 (2014).
- Rao, C. N. R., Gopalakrishnan, J. New Directions in Solid State Chemistry. , Cambridge University Press. (1989).
- Zhu, H., Rosenfeld, D. C., Anjum, D. H., Caps, V., Basset, J. -M. Green Synthesis of Ni-Nb Oxide Catalysts for Low-Temperature Oxidative Dehydrogenation of Ethane. ChemSusChem. 8, 1254-1263 (2015).
- Heracleous, E., Lemonidou, A. A. Ni-Nb-O Mixed Oxides as Highly Active and Selective Catalysts for Ethene Production via Ethane Oxidative Dehydrogenation. Part I: Characterization and Catalytic Performance. J. Cat. 237, 162-174 (2006).
- Savova, B., Loridant, S., Filkova, D., Millet, J. M. M. Ni-Nb-O Catalysts for Ethane Oxidative Dehygenation. Appl. Catal. A. 390 (1-2), 148-157 (2010).
- Heracleous, E., Delimitis, A., Nalbandian, L., Lemonidou, A. A. HRTEM Characterization of the Nanostructural Features formed in Highly Active Ni-Nb-O Catalysts for Ethane ODH. Appl. Catal. A. 325 (2), 220-226 (2007).
- Skoufa, Z., Heracleous, E., Lemonidou, A. A. Unraveling the Contribution of Structural Phases in Ni-Nb-O mixed oxides in Ethane Oxidative Dehydrogenation. Catal. Today. 192 (1), 169-176 (2012).
- Heracleous, E., Lemonidou, A. A. Ni-Me-O Mixed Metal Oxides for the Effective Oxidative Dehydrogenation of Ethane to Ethylene - Effect of Promoting Metal Me. J. Cat. 270, 67-75 (2010).
- Zhu, H., et al. Nb Effect in the Nickel Oxide-Catalyzed Low-Temperature Oxidative Dehydrogenation of Ethane. J. Cat. 285, 292-303 (2012).
- Sadovskaya, E. M., et al. Mixed Spinel-type Ni-Co-Mn Oxides: Synthesis, Structure and Catalytic Properties. Catal. Sustain. Energy. 3, 25-31 (2016).
- Alvarez, J., et al. Ni-Nb-Based Mixed Oxides Precursors for the Dry Reforming of Methane. Top. Catal. 54, 170-178 (2011).
- Jin, S., Guan, W., Tsang, C. -W., Yan, D. Y. S., Chan, C. -Y., Liang, C. Enhanced hydroconversion of lignin-derived oxygen-containing compounds over bulk nickel catalysts though Nb2O5 modification. Catal. Lett. 147, 2215-2224 (2017).
- Taghavinezhad, P., Haghighi, M., Alizadeh, R. CO2/O2-oxidative dehydrogenation of ethane to ethylene over highly dispersed vanadium oxide on MgO-promoted sulfated-zirconia nanocatalyst: Effect of sulfation on catalytic properties and performance. Korean J. Chem. Eng. 34 (5), 1346-1357 (2017).
- Muralidharan, G., Subramanian, L., Nallamuthu, S. K., Santhanam, V., Kumar, S. Effect of Reagent Addition Rate and Temperature on Synthesis of Gold Nanoparticles in Microemulsion Route. Ind. Eng. Chem. Res. 50 (14), 8786-8791 (2011).
- Sosa, Y. D., Rabelero, M., Treviño, M. E., Saade, H., López, R. G. High-Yield Synthesis of Silver Nanoparticles by Precipitation in a High-Aqueous Phase Content Reverse Microemulsion. J. Nanomater. , 1-6 (2010).
- Morterra, C., Cerrato, G., Pinna, F. Infrared spectroscopic study of surface species and of CO adsorption: a probe for the surface characterization of sulfated zirconia catalysts. Spectrochim. Acta. A Molecul. Biomolecul. Spectrosc. 55, 95-107 (1998).
- Yang, F., Wang, Q., Yan, J., Fang, J., Zhao, J., Shen, W. Preparation of High Pore Volume Pseudoboehmite Doped with Transition Metal Ions through Direct Precipitation Method. Ind. Eng. Chem. Res. 51 (47), 15386-15392 (2012).
- Saleh, R., Djaja, N. F. Transition-metal-doped ZnO nanoparticles: Synthesis, characterization and photocatalytic activity under UV light. Spectrochim. Acta. A Molecul. Biomolecul. Spectrosc. 130, 581-590 (2014).
- Ertis, I. F., Boz, I. Synthesis and Characterization of Metal-Doped (Ni, Co, Ce, Sb) CdS Catalysts and Their Use in Methylene Blue Degradation under Visible Light Irradiation. Modern Research in Catalysis. 6, 1-14 (2017).
- Jin, S., et al. Cleavage of Lignin-Derived 4-O-5 Aryl Ethers over Nickel Nanoparticles Supported on Niobic Acid-Activated Carbon Composites. Ind. Eng. Chem. Res. 54 (8), 2302-2310 (2015).
- Rojas, E., Delgado, J. J., Guerrero-Pérez, M. O., Bañares, M. A. Performance of NiO and Ni-Nb- O Active Phases during the Ethane Ammoxidation into Acetonitrile. Catal. Sci. Technol. 3 (12), 3173-3182 (2013).
- Lee, S. -H., et al. Raman Spectroscopic Studies of Ni-W Oxide Thin Films. Solid State Ionics. 140 (1), 135-139 (2001).
- Mondal, A., Mukherjee, D., Adhikary, B., Ahmed, M. A. Cobalt nanoparticles as recyclable catalyst for aerobic oxidation of alcohols in liquid phase. J. Nanopart. Res. 18 (5), 1-12 (2016).
- Wang, K., Yang, L., Zhao, W., Cao, L., Sun, Z., Zhang, F. A facile synthesis of copper nanoparticles supported on an ordered mesoporous polymer as an efficient and stable catalyst for solvent-free sonogashira coupling Reactions. Green Chem. 19, 1949-1957 (2017).
- Song, Y., et al. High-Selectivity Electrochemical Conversion of CO2 to Ethanol using a Copper Nanoparticle/N-Doped Graphene Electrode. Chemistry Select. 1, 6055-6061 (2016).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone