Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Sperm Collection of Differential Quality Using Density Gradient Centrifugation
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
In this paper, we aim to describe the performance of the density gradient centrifugation technique and its application in sperm physiology research.
Streszczenie
In sexual reproduction, a male gamete or sperm cell fuses with a female gamete to bring about fertilization. However, a large number of sperm cells with fertilizing ability are required to interact with a female gamete to ensure fertilization. As such, the fertilizing ability of individual sperm cells is critical for successful reproduction. Density gradient centrifugation has been utilized for several decades as a reproducible, fast, efficient, effective and extremely adaptable method to collect only high-quality sperm to be used in assisted reproductive technology. The protocols we described herein focus on the utilization of the discontinuous Percoll density gradient centrifugation (PDGC) technique to isolate three distinct populations of rooster sperm by their quality. We were able to collect low-, medium- and high-quality sperm. We also describe reproducible protocols that entail determining fertility potential of sperm by assessing their viability, mobility and penetrability. Collection of sperm by their quality using PDGC technique would be useful to accurately and thoroughly characterize sperm with differential fertility potential.
Wprowadzenie
In vertebrates, male gametes undergo intense selective pressure; therefore reproductive fitness of a male is pivotal for achieving successful fertilization. Males of any given vertebrate species must be able to produce sperm cells in large quantities and of sufficient quality in order to meet the needs of fertilization. Sperm cells, having both a sperm head and a flagellum, are the most polarized cells in the body. They are also very heterogeneous in quality of sperm (live and dead, morphologically normal and abnormal, and immobile, low mobile and high mobile), which is revealed through the wide variation in reproductive efficiency of the males. The larger the proportion of high-quality sperm, the fewer the number of matings required to successfully fertilize the ovum. However, to achieve fertility, morphologically normal sperm cells rely on propulsive forces generated by their flagella to reach the site of fertilization as well as to penetrate the zona pellucida1 (ZP; in the case of mammals) or inner perivitelline layer2 (IPVL; in the case of birds and reptiles) of the ovum following natural mating or artificial insemination (AI). Determining sperm quality is necessary for use in assisted reproductive technologies3 (ART) and selection of breeding males to be used in AI programs4. On the other hand, the success of ART solely relies upon the accurate evaluation of sperm quality. A number of laboratory tests have been developed to determine the functional characteristics of sperm. The most important parameters are sperm morphology, viability, mobility, capacitation (avian sperm do not require capacitation5), acrosome reaction (AR; exocytosis and release of a proteolytic enzyme from the acrosome of the sperm head), sperm penetration of ZP or IPVL, and fertilization6,7,8,9,10,11. Measures of fertility alone do not provide an accurate evaluation of the fertilizing ability of a sperm population11. Measures of the several events leading up to fertilization of an egg allow for an appropriate representation of the performance of individual spermatocytes7.
The methodology developed for measuring sperm function is primarily species-specific. For example, in avian sperm, viability, mobility and penetration of IPVL are the most common parameters used to assess sperm quality8,11,12. The number of live sperm in the ejaculate plays a crucial role for the survival of sperm because the presence of a large number of dead sperm in the semen affects the quality of sperm. This enhances the production of reactive oxygen species in the semen and causes oxidative damage to the live sperm13. Sperm mobility, the capacity for flagellar movement of avian sperm against resistance at body temperature, is known to play a direct role in bringing about fertilization8. It is well established that mobility is positively correlated with fertility and is, therefore, a primary determinant of fertility8. However, a mobile sperm must also have the ability to undergo an AR and to penetrate the IPVL11. IPVL penetration assays take account for every sperm that participates in the process of fertilization11.
In the application of ART, ejaculate is usually processed in order to maximize the concentration of high-quality sperm and minimize concentration of low-quality sperm. After collection of semen, the proportion of high-quality sperm can be enriched through sperm separation procedures commonly used in both industry and research practices. Many of these procedures have been developed, all with respective benefits and limitations, but all utilize the heterogeneous nature of sperm to collect only the sperm with high fertilizing ability. These procedures include sperm migration methods, adherence column filtration and density gradient centrifugation (DGC)14,15,16,17,18,19,20. Among the available techniques, DGC has been found to be very simple, repeatable, cost-effective and efficient in isolating the maximum amount of high-quality sperm for use in ART with the goal of maximizing chance of fertilization14,15. In addition, DGC is not injurious to the sperm cell membrane. In contrast, sperm migration methods collect only progressively mobile sperm18,19, but the quantity of sperm collected is very low, making it inefficient in collecting large volumes of sperm18,20. Adherence column filtration is very efficient in filtering highly mobile sperm from semen17; however, it tends to be injurious to sperm membranes20,21.
In the DGC technique, the most commonly used substrate for generating the density gradient is Percoll, which consists of colloidal silica particles coated in polyvinylpryrolidone. Percoll density gradient centrifugation (PDGC) can either be continuous or discontinuous but a discontinuous gradient is most commonly used for high yield isolation of highly mobile sperm13,16,20. In a discontinuous gradient, lower density media floats above higher density media, creating a gradient that increases in density from the top to the bottom of a conical tube. This creates boundaries at the interface between the two media of differing density. The efficiency of PDGC is derived from two factors: 1) the propulsive ability of individual sperm cells and 2) the tendency of sperm cells with high structural integrity to have an increased density. Sperm with higher mobility are better able to cross from lower density media and penetrate into a higher density media. Lower mobility sperm are more likely to become trapped at the boundary created by the interface between media of differing density. Sperm cells with high structural integrity and mobility tend to have a higher density than dead, abnormal or low mobile sperm cells. When centrifugal force is applied in PDGC, this facilitates movement of sperm with different densities to their respective place in the gradient.
In general practice, after PDGC is performed, the soft pellet of sperm with high fertility potential at the bottom of the conical tube is collected, and the remainder is discarded. However, an underutilized advantage of this technique is its ability to separate sperm cells into several groups based on the quality differences. For research purposes, separation of sperm by degree of quality utilizing the PDGC technique allows for study of sperm quality as it pertains to physiologic, metabolomic and proteomic differences. Here, we aim to detail how this technique may be used to separate sperm by quality, as well as demonstrate these differences in quality, using the previously established eosin-nigrosin vital staining for viability, Accudenz assay for mobility, and sperm-IPVL interaction assay for penetrability.
Protokół
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Georgia.
1. Washing using Traditional Centrifugation
- Prepare phosphate buffer solution (PBS). Add 8.0 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4 and 0.24 g of KH2PO4 to 800 mL of distilled water (dH2O). Adjust the pH to 7.4 using 0.1 N HCl and bring the solution to 1 L using dH2O.
- Prepare motility buffer. Add 6.5 g of NaCl, 4.5 g of glucose, 0.444 g of CaCl2 and 11.5 g of N-tris-[hydroxymethyl] methyl-2-amino-ethanesulfonic acid (TES) to 800 mL of dH2O. Adjust the pH to 7.4 using 1 M NaOH and bring the solution to 1 L using dH2O.
- Pipette 0.5 mL of semen into a polypropylene microcentrifuge tube. Add 1.0 mL of PBS and mix gently.
- Centrifuge at 1,500 x g for 10 min at room temperature (RT) and discard the supernatant. Resuspend the sperm pellet with PBS up to 1.5 mL.
- Centrifuge at 1,500 x g for 10 min at RT. Resuspend the sperm pellet with motility buffer up to 0.5 mL.
2. Performing the PDGC technique
NOTE: Perform the entire process of PDGC at room temperature.
- Make 3.0 mL of 1.08 g/mL and 1.07 g/mL Percoll solutions in two separate tubes.
- In a clean test tube, add 1.712 mL of the 1.13 g/mL original Percoll to 0.3 mL of 1.5 M NaCl solution. Add 0.988 mL of dH2O and mix by gentle inversion to make 3.0 mL of a 1.08 g/mL density solution.
- In a clean test tube, add 1.482 mL of the 1.13 g/mL original Percoll to 0.3 mL of 1.5 M NaCl solution. Add 1.218 mL of dH2O and mix by gentle inversion to make 3.0 mL of a 1.07 g/mL density solution.
- In a clean test tube, dilute 1.0 mL of semen sample 1:2 with 2.0 mL of PBS. Mix gently by pipetting.
- Pipet 3.0 mL of the 1.07 g/mL density solution into a sterile 15 mL conical tube. Carefully pipet 3.0 mL of the 1.08 g/mL density solution beneath the 1.07 g/mL density solution. Ensure that the two layers do not mix. A long-form (9 in) Pasteur pipette can make this step easier.
- Pipet 3.0 mL of diluted semen sample overtop the PDG. To ensure that the semen sample does not mix with the PDG, gently tilt the conical tube containing the PDG at a 45° angle. Pipet the sample along the wall of the tube and allow it to flow down the tube and over the PDG.
- Prepare a blank tube to match the mass of the PDG with overlaid sample. Centrifuge both tubes at 1500 x g for 20 min. Be careful to maintain the discontinuous gradient while transferring the tubes from the bench to the balance and then to the centrifuge.
NOTE: Do not use the brake at the end of centrifugation. - Observe the results. Ensure that three distinct semen layers have formed in the tube, as seen in Figure 1.
- Aspirate isolated semen layers with a pipette. Collect the top layer of semen first, the middle layer second, and last the hard pellet at the bottom of the tube. Transfer each to a clean and sterile polypropylenemicrocentrifuge tube.
- Dilute each sample to 1.5 mL with PBS. Centrifuge at 1500 x g for 10 min.
- Pour off the supernatant. Reconstitute sperm pellet with motility buffer by gentle pipetting.
NOTE: Alternative densities may be used to suit investigator needs. Determine amounts of ingredients used with the following equation, where v0 is volume of stock density solution used, v is final volume of solution desired, p is density of final density solution desired and p0 is the density of the stock density solution:
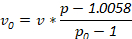
Always use 0.3 mL of 1.5 M NaCl in preparation of density solutions to match the NaCl concentration of physiological saline.
3. Determining Sperm Quality
- Calculate the sperm concentration as previously described22.
- Perform eosin-nigrosin vital staining as previously described12 with the following modifications:
- Prepare 100 µL of sperm solution at a concentration of 1 x 108 cells/mL.
- Pipet 50 µL of sperm solution in a polypropylene microcentrifuge tube containing an equal volume of eosin-nigrosin stain. Incubate the mixture for 5 min at room temperature.
- Place a 20 µL drop of stained sperm sample at one end of a glass slide and smear uniformly in a manner similar to that used for blood smears. Air-dry the smeared slides at room temperature for 3-5 min.
- Observe the smear under microscope. Count the number of live sperm (no stain) and dead sperm (stained pink) and calculate the percentage of live sperm.
- Perform the Accudenz assay, as previously validated for the chicken sperm, to objectively assess the sperm mobility8 with the following modifications:
- Pipet 1.0 mL of 6% assay solution into polystyrene cuvettes, as illustrated in Figure 2. Incubate to 41 °C.
NOTE: 41 °C is used to match the internal temperature of a hen. The incubation temperature should match that of the female reproductive tract of the species being investigated. - Overlay the preheated assay solution with 100 µL of semen sample at a concentration of 5 x 108 cells/mL.
- Place the cuvette containing overlaid sperm sample in the spectrophotometer. Record the absorbance value at 550 nm.
- Pipet 1.0 mL of 6% assay solution into polystyrene cuvettes, as illustrated in Figure 2. Incubate to 41 °C.
- Perform IPVL-penetration assay as previously described11 with the following modifications:
- Cut a piece (0.5 cm x 0.5 cm) of non-germinal disc region of intact IPVL.
- Adjust sperm concentration to 4 x 106 cells/mL
- Incubate sperm in motility buffer with IPVL in a small glass vial for 15 min at 37 °C, as illustrated in Figure 3.
- Immerse the IPVL piece in 3% NaCl to stop the interaction between the IPVL and sperm.
- Mount the IPVL piece on a microscope slide and stain with Schiff's reagent for 10 min following fixation with 10% formalin for 20 s.
- Observe the IPVL under a microscope for successful sperm penetration holes and count the number of all visible holes per 0.25 mm2 at 40X magnification.
Wyniki
The PDGC technique resulted in distinct separation of three layers of sperm by degree of quality across all parameters. Sperm separates into a high-quality layer below the higher density solution, a medium-quality layer between the higher and lower density solution and a low-quality layer above the lower density solution. These differences in quality are evidenced by clear differences in viability (Figure 4), mobility (Figure 5) ...
Dyskusje
Fertility not only determines the profitability of animal production but also acts as a means of natural selection of species for existence. The ultimate function of a sperm cell is to fertilize an ovum. The oviduct of a female selects only those fittest sperm in order to ensure fertilization of the ovum23,24. In vitro studies have also revealed a close correlation between qualitative sperm traits and fertilization success4,<...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
None.
Materiały
| Name | Company | Catalog Number | Comments |
| Accudenz | Accurate Chemical and Scientific Corporation, Westbury, NY, USA | AN7050 | |
| Percoll | Sigma-Aldrich, Corp., St. Louis, MO, USA | P7828 | |
| Schiff’s reagent | Sigma-Aldrich, Corp., St. Louis, MO, USA | 3952016 | |
| TES | Sigma-Aldrich, Corp., St. Louis, MO, USA | T1375 | |
| Eosin Y | Sigma-Aldrich, Corp., St. Louis, MO, USA | E4009 | |
| Nigrosin | Sigma-Aldrich, Corp., St. Louis, MO, USA | 198285 | |
| ST 40R Centrifuge | Thermo Scientific, Waltham, MA, USA | 75004524 | |
| DU 530 Life Sciences UV/Vis Spectrophotometer | Beckman Coulter, Brea, CA, USA | No catalogue is found | |
| Olympus IX 71 Inverted Fluorescence and Phase Contrast Microscope | Olympus America Inc., PA, USA | No catalogue is found |
Odniesienia
- Spargo, S. C., Hope, R. M. Evolution and nomenclature of the zona pellucida gene family. Biology Reproduction. 68, 358-362 (2003).
- Okamura, F., Nishiyama, H. The passage of spermatozoa through the vitelline membrane in the domestic fowl, Gallus gallus. Cell and Tissue Research. 188 (3), 497-508 (1978).
- Henkel, R., et al. Sperm function and assisted reproduction technology. Reproductive Medicine and Biology. 4, 7-30 (2005).
- Reddy, R. P., Bakst, M. R., Wishart, G. J. Artificial Insemination of broilers: Economic and management implications. Proceedings of 1st International Symposium on Artificial Insemination of Poultry. Poultry Science Association. , 73-89 (1995).
- Howarth, B. An Examination for Sperm Capacitation in the Fowl. Biology of Reproduction. 3, 338-341 (1971).
- Menkveld, R., Holleboom, C. A. G., Rhemrev, J. P. T. Measurement and significance of sperm morphology. Asian Journal of Andrology. 13, 59-68 (2011).
- Kumaresan, A., Johannisson, A., Al-Essawe, E. M., Morrell, J. M. Sperm viability, reactive oxygen species, and DNA fragmentation index combined can discriminate between above- and below-average fertility bulls. Journal of Dairy Science. 100, 5824-5836 (2017).
- Froman, D. P., McLean, D. J. Objective measurement of sperm motility based upon sperm penetration of Accudenz. Poultry Science. 75, 776-784 (1996).
- Zaneveld, L. J., De Jonge, C. J., Anderson, R. A., Mack, S. R. Human sperm capacitation and the acrosome reaction. Human Reproduction. 6 (9), 1265-1274 (1991).
- Ahammad, M. U., et al. Acrosome reaction of fowl sperm: Evidence for shedding of acrosomal cap in intact form to release acrosomal enzyme. Poultry Science. 92 (3), 798-803 (2013).
- Ahammad, M. U., et al. Maturational changes in motility, acrosomal proteolytic activity, and penetrability of the inner perivitelline layer of fowl sperm, during their passage through the male genital tract. Theriogenology. 76 (6), 1100-1109 (2011).
- Chalah, T., Brillard, J. P. Comparison of assessment of fowl sperm viability by eosin-nigrosin and dual fluorescence. Theriogenology. 50 (3), 487-493 (1998).
- Aitken, R. J., West, K. M. Analysis of the relationship between reactive oxygen species production and leukocyte infiltration in fractions of human semen separated on Percoll gradients. International Journal of Andrology. 13, 433-451 (1990).
- Mortimer, D., Mortimer, S. T., Carrell, D., Aston, K. Density Gradient Separation of Sperm for Artificial Insemination. Spermatogenesis. Methods in Molecular Biology (Methods and Protocols. , 927 (2013).
- Qingling, Y., et al. Processing of semen by density gradient centrifugation selects spermatozoa with longer telomeres for assisted reproduction techniques. Reproductive BioMedicine Online. 31, 44-50 (2015).
- Moohan, J. M., Lindsay, K. S. Spermatozoa selected by a discontinuous Percoll density gradient exhibit better motion characteristics, more hyperactivation, and longer survival than direct swim-up. Fertility and Sterility. 64 (1), 160-165 (1995).
- Paulson, J. D., Polakoski, K. L. A glass wool column procedure for removing extraneous material from human ejaculate. Fertility and Sterility. 28, 178-181 (1977).
- Ahammad, M. U., Chiaki, N., Tatemoto, H., Kawamoto, Y., Nakada, T. Utilization of the swim-up migration sedimentation technique to separate viable and progressively motile fowl spermatozoa. World's Poultry Science Association Proceedings. , (2010).
- Lucena, E., et al. Recovery of motile sperm using the migration-sedimentation technique in an in vitro fertilization-embryo transfer programme. Human Reproduction. 4 (2), 163-165 (1989).
- Henkel, R. Sperm Processing for IVF. Clinical Embryology: A Practical Guide. , (2013).
- Sherman, J., Paulson, D., Liu, K. Effect of glass wool filtration on ultrastructure of human spermatozoa. Fertility and Sterility. 36, 643-647 (1981).
- Freund, M., Carol, B. Factors affecting haemocytometer counts of sperm concentration in human semen. Journal of Reproduction and Fertility. 8, 149-155 (1964).
- Bakst, M. R., Wishart, G. J., Brillard, J. P. Oviducal sperm selection, transport and storage in poultry. Poultry Science Review. 5, 117-143 (1994).
- Ahammad, M. U., Okamoto, S., Kawamoto, Y., Nakada, T. The effects of regular fluid secretion from the uterus of laying hens on the longevity and fertilization ability of fowl sperm in the oviduct. Poultry Science Journal. 1 (1), 13-22 (2013).
- Ahammad, M. U., et al. Maturational changes in the survivability and fertility of fowl sperm during their passage through the male reproductive tract. Animal Reproduction Science. 128 (1-4), 129-136 (2011).
- Choi, K. H., Emery, D. A., Straub, D. E., Lee, C. S. Percoll process can improve semen quality and fertility in turkey breeders. Asian-Australasian Journal of Animal Sciences. 12 (5), 702-707 (1999).
- Chen, M. J., Bongso, A. Comparative evaluation of two density gradient preparations for sperm separation for medically assisted conception. Human Reproduction. 14 (3), 759-764 (1999).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone