Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Behavioral Approaches to Studying Innate Stress in Zebrafish
W tym Artykule
Podsumowanie
This manuscript describes a simple method to measure stress behaviorally in adult zebrafish. The approach takes advantage of the innate tendency that zebrafish prefer the bottom half of a tank when in a stressful state. We also describe methods for coupling the assay with pharmacology.
Streszczenie
Responding appropriately to stressful stimuli is essential for survival of an organism. Extensive research has been done on a wide spectrum of stress-related diseases and psychiatric disorders, yet further studies into the genetic and neuronal regulation of stress are still required to develop better therapeutics. The zebrafish provides a powerful genetic model to investigate the neural underpinnings of stress, as there exists a large collection of mutant and transgenic lines. Moreover, pharmacology can easily be applied to zebrafish, as most drugs can be added directly to water. We describe here the use of the 'novel tank test' as a method to study innate stress responses in zebrafish, and demonstrate how potential anxiolytic drugs can be validated using the assay. The method can easily be coupled with zebrafish lines harboring genetic mutations, or those in which transgenic approaches for manipulating precise neural circuits are used. The assay can also be used in other fish models. Together, the described protocol should facilitate the adoption of this simple assay to other laboratories.
Wprowadzenie
Stress responses are altered behavioral and physiological states resulting from potentially harmful or aversive stimuli. Stress responses are conserved throughout the animal kingdom, and are critical for the survival of an organism1. Decades of research have greatly expanded our knowledge of some of the genetic and neuronal mechanisms underlying stress states. Today, areas of the brain such as the amygdala and the striatum2, and genetic factors such as corticotropin releasing hormone (crh), and the glucocorticoid (gr) and mineralocorticoid receptors (mr) have been studied extensively3,4,5,6. Despite these critical findings, much remains unknown about genetic and neuronal regulation of stress. As such, many stress related disorders suffer from a lack of therapeutics.
Genetically amendable model organisms provide a useful tool in the study of genetic and neuronal control of behavior. Fish models, in particular, are extremely powerful: they are small organisms with short generation times, their use in a laboratory setting is facile, their genomes are easily modified, and, as a vertebrate, they share not only genetic, but also neuroanatomical homology with their mammalian counterparts7,8. Standard assays for measuring stress can be paired with zebrafish lines harboring genetic mutations, or those in which manipulation of precise neuronal subsets is possible, and the effects of single genes or defined neurons can be assessed rapidly and efficiently.
Behaviorally, stress responses can be characterized in fish as periods of hyper-activity or prolonged periods of inactivity (akin to 'freezing')9, reduced exploration10, rapid breathing, reduced food intake11, and a place-preference for the bottom of a tank12. For example, when placed into an unfamiliar tank, adult zebrafish and other small fish models show an initial preference for the bottom half of the tank, yet, over time, the fish begin exploring top and bottom halves with near-equal frequency12. Treatment of adults with drugs known to reduce anxiety cause fish to explore immediately the top half10,13. Conversely, drugs that increase anxiety cause fish to show strong preference for the bottom half of the tank12,14,15. Thus, reduced exploration and preference for the bottom half of the tank are simple and reliable indicators of stress.
Like most vertebrates, stress responses in fish are driven by activation of hypothalamic-pituitary-inter-renal axis (HPI; analogous to the hypothalamic-pituitary-adrenal [HPA] axis in mammals)14,16. Hypothalamic neurons expressing the hormone corticotropin-releasing hormone (CRH) signal to the pituitary, which in turn releases adrenocorticotropic releasing hormone (ACTH). ACTH then signals to the inter-renal gland to produce and secrete cortisol, which has a number of downstream targets16, one of them being negative feedback of the crh-producing hypothalamic neurons3,17,18,19.
Here, we describe a method to assess behavioral measures of innate stress. For the behavior, we detail protocols using the novel tank diving test12,14. We then demonstrate, as an example, that a known anxiolytic drug, buspirone, reduces behavioral measures of stress.
Protokół
The protocol has been approved by the Institutional Animal Care and Use Committeeat Florida Atlantic University .
1. Preparation
- Designate an isolated room for performing behavioral studies, or close off a section of a room so that it is isolated.
NOTE: The room should be undisturbed and have low traffic to avoid disrupting normal behavior of the fish. - Move the following materials and equipment into the behavioral room: (i) a camera and lens, (ii) an infrared filter which can be attached to the lens, (iii) a camera stand, (iv) a computer with camera acquisition software, (v) a firm and stable table to perform the assay on, (vi) infrared lights (IR lights; 850 nm or 940 nm), (vii) a white acrylic diffuser, which is longer than the length of the recording tank (viii) 1.8 L trapezoidal plastic assay tank (referred to as the 'novel tank'; the one used here is 12 x 18 inches), and (ix) a bucket of fish system water.
NOTE: For the novel tank, our lab uses commercially available plastic vessels, which are trapezoidal in shape. The dimensions of the tank are roughly 6 in x 9 in (detailed dimensions are provided in Figure 1A). The diffuser board we use is slightly larger than the novel tank (12 in x 18 in). Novel tank experiments have been performed with tanks having differing shapes, such as those which are rectangular or those with different trapezoidal dimensions20,21. Typically, fish behavior is similar in all tanks regardless of their dimensions: for all containers, fish initially prefer the bottom half, yet over time begin exploring top half with greater frequency. - Attach the infrared filter to the camera lens. The wavelengths of the infrared light strips typically range from 850 nm to 940 nm. The filter is a long pass filter that restricts light of wavelengths less than 720 nm from transmitting through to the camera.
- Select suitable parameters for the camera acquisition software. For most recordings, set the camera acquisition to a rate of 30 frames-per-second, and recording duration to 10-min.
NOTE: These parameters may differ, depending on the experiment. For example, to study habituation in a novel tank22,23, longer recordings may be required.
2. Setup
NOTE: The steps in this section describe setting up the novel tank assay. A diagram of the end product is given in Figure 1B.
- Place the novel tank in the middle of the table.
- Position the infrared lights behind the tank and place the white acrylic sheet or diffuser screen in between the tank and LED light source.
- Place the diffuser so that it maximally spreads the light coming from the LEDs, and the intensity of the light is enough to illuminate the novel tank. The closer the board is to the light source, the brighter the lights will be, yet the less it will diffuse. By contrast, placing the diffuser board away from the light source will reduce light intensity, but spread the light better.
- Fill approximately three quarters of the novel tank with fish system water.
NOTE: System water is generated using reverse osmosis of tap water, followed by dosing such that conductivity equals 900 ± 100 µS, that pH is neutral (7.2), and that the temperature is 27 ± 1 °C. - Attach the camera to the camera stand and connect the camera to the computer. Open up the video acquisition software and adjust the camera to face the front of the tank and ensure the entire novel tank can be seen and that there are no obscured areas in the video. Adjust the tank and the infrared lights such that there is sufficient and even illumination throughout the tank when observed through the camera.
NOTE: Before proceeding to experiments, it can often be useful to perform a trial run, in which video of a fish is captured and tracking is performed. This will ensure that the setup is sufficient for experimentation of behavior.
3. Novel tank test setup
- Prepare a 250 mL beaker pre-filled with fish system water, and at least two holding tanks.
- On the morning of the test, transfer at least 10 test adult zebrafish to be used for each experimental condition (controls and experimental adults) from fish facility into a holding tank, transfer them to the behavior room, and allow them to acclimate for at least one hour.
NOTE: A power analysis should be performed before experimentation, yet in our hands, an n = 10 is usually sufficient to detect statistical significance. Moreover, the holding tank should contain no more than five individuals per liter of water. An acclimation of one hour is sufficient as zebrafish adults have been shown to habituate within 30 minutes of a new tank22. Also, behavioral rhythms are affected by circadian processes, and thus experimental replicates done on different days should be performed within the same hours. We typically perform all experiments between the hours of 11:00 am and 6:00 pm. - Label the tanks such that the condition or genotype of the animals is blind to the experimenter.
NOTE: Experiments can easily be blinded to the experimenter by labeling tanks using a letter or number system (i.e., one tank is labeled 'A', another 'B', etc.). A party not involved in the experiments labels the tanks with such a system, and masks the identities from the experimenter until after post-analysis is complete. - Using a net, gently place a single adult in the pre-filled beaker from step 3.1. Allow the adult fish to acclimate in the beaker for 10 minutes.
NOTE: Record the sex of the adult, as it might be important post-analysis to look for sex-specific differences. - After acclimation in the beaker, introduce the fish into the novel tank (set up in section 1) by gently pouring out the water and adult from the beaker.
- After introducing the adult into the novel tank, start the camera recording, and move away from the setup to prevent additional distress to the fish.
- After the recording has finished, remove the individual from the novel tank and place into a new holding tank.
NOTE: A different holding tank from the one in step 3.2 should be used to prevent repeated testing on the same individuals. - Repeat steps 3.4 to 3.7 for each adult until all animals have been tested.
NOTE: In addition to blinding conditions or genotypes, randomize trials. Use a random number generator or any tool that allows one to randomize between the trials. This should be done before experimentation so that each trial is determined before the experiments begin. - At the end of all tests, return the fish back to the fish facility.
4. Pretreatment with drug
NOTE: The aim of the following steps is to compare the behavior of an individual before and after the use of drugs. This comparison is achieved by first performing a novel tank test as in step 3.4 to 3.6, followed by drug treatment, and then a second novel tank test (Figure 3A).
- Prepare a stock solution of the drug, including positive and negative controls.
NOTE: If the drug has previously been used in the literature, find an appropriate working dose and use this. For example, for buspirone in the representative results, we make a 100x stock solution and use 0.05 mg/mL as the final concentration, as described in the literature13,20. If suggested dose is unknown, a dose response curve should be performed by examining several concentrations. Set up more beakers with serial dilutions of drug. If the drug is not dissolvable in water, use dimethyl sulfoxide (DMSO) as a solvent. - Dilute drugs to working concentration in 250 mL beakers with system water. For example, if a 100x solution was made, dilute 1:100 in system water. Set up a beaker with only system water as a control.
NOTE: If DMSO was used as a solvent in step 3.1, use an equal volume of DMSO in the control beaker. - With the help of another researcher, mask the identities of the drug and control beakers to ensure that the tester is blind to the treatment conditions until post-analysis.
NOTE: A number or letter system may be used. - Perform a novel tank test by following steps 3.1 to 3.6 to obtain a baseline behavioral stress response.
- After the baseline recording, use a net to immediately remove the adult fish from the novel tank and place it in the beaker dosed with drug or vehicle, as described in step 4.2. Allow the adult to stay in the beaker for 10 min.
NOTE: Ensure that the net does not touch the water in the beakers to prevent cross-contamination of drugs. Ensure proper dosage and administration time depending on the drug used. A 10 min treatment time might not work for all drugs. - After treatment, use a net to remove the adult from the beaker in step 4.5 and place it in another beaker filled with fresh system water only. This is the washout period to minimize further dosing during the second novel tank test. Allow the adult to stay in the wash out beaker for an additional 10 min.
NOTE: Separate nets should be used for each drug condition to prevent any unwanted cross treatment with drug. The washout period may be skipped if the experimenter wishes. - Perform the novel tank diving test a second time by removing that adult from the beaker in previous step, place it in a new novel tank, and follow steps 3.5 to 3.6.
- After the second novel tank test, remove the individual into a separate holding tank. Pour away the system water in the second novel tank and fill it with fresh system water for the next test. This step prevents cross-contamination of any drug.
NOTE: Depending on the half-life of the drugs, fresh beakers containing drugs should be made every 3 hours. For buspirone, make fresh solutions every 3 hours. In addition, following the note in step 3.8, the trials should be randomized between controls and drug treatments. - At the end of all trials, return individuals back into the fish facility.
NOTE: Depending on type of drug used, the effects of these treatments on individuals can be long lasting. Therefore, do not use these individuals in other experiments.
5. Video analysis
- After all trials, load video files into tracking software of choice.
NOTE: We typically use commercially available tracking software, yet several freely available software packages can be used. The steps to achieve tracking may differ according to the software package used. - Using a still frame from the video, define imaginary boundaries around (i) the entire novel tank arena that is filled with water, and boundaries around (ii) the top third, (iii) middle third, and (iv) bottom third of the tank. Use these to establish time that the fish spent in each portion of the novel tank.
- Calculate x-y displacement per frame for each arena defined in step 5.2.
- Define top, middle, and bottom areas of the tank. Each region should be similar in size. A brief method for determining these regions is to calculate the length of the tank in the y-direction, and divide this by 3.
NOTE: Variations to the general protocol do exist. For example, some labs use halves instead of thirds14. - Determine the time spent in each arena, the distance, and the velocity.
NOTE: Most tracking packages will automatically calculate these for the user. However, if the tracking software does not, these can be calculated easily from the x-y displacement values. For example, distance can be determined using the formula:
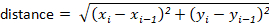
and velocity can be determined following the formula:
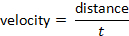
- Repeat steps 5.2 to 5.4 to acquire tracks and measurements for all trials.
NOTE: Variations to this general protocol
6. Testing for normality
- Perform statistics before proceeding to calculate statistical differences. Check to see if the data is normally distributed using a Shapiro-Wilk test.
- If the null hypothesis that the data is normally distributed is rejected (i.e., the data does not follow a Gaussian distribution), perform all tests using non-parametric tests. Conversely, if the data is found to follow a normal distribution, proceed to use parametric tests.
NOTE: We use commercially available software to perform statistics; however, the R programming language can also be used. A Shapiro-Wilk analysis can be performed using R's shapiro.test function.
Wyniki
Examining stress in zebrafish
To examine stress behavior over time in wild-type zebrafish, we tested adult fish from the AB strain24 in the novel tank test. AB adults were subjected to the protocol as described above. Briefly, fish were given a 1-h acclimation period in a tank in the behavior room. An individual was placed in a beaker for 10-min, and then placed gently in an unfamiliar tank (novel tank) filled with fresh system water. Locomotor activ...
Dyskusje
Zebrafish exhibit a robust stress response in a novel tank
Here, we describe a simple behavioral approach for examining stress responses in adult zebrafish, and validate the approach as a simple measure of stress using pharmacology.
The novel tank test is a widely used test for examining innate stress in zebrafish and other species of fish12,14,21,35
Ujawnienia
The authors declare that they have no competing or financial interests.
Podziękowania
This work was supported by funding from the Jupiter Life Science Initiative at Florida Atlantic University to ERD and ACK. This work was also supported by grants R21NS105071 (awarded to ACK and ERD) and R15MH118625 (awarded to ERD) from the National Institutes of Health.
Materiały
| Name | Company | Catalog Number | Comments |
| Camera | We use Point Grey Grasshopper3 USB camera with lens from Edmund Optics. | ||
| Infrared filter | Edmund Optics | ||
| Video Acquisition Program | Use programs such as Virtualdub or FlyCapture because the acquisition framerate can be set. | ||
| Infrared LED lights | |||
| Assay tank | Aquaneering | Part number ZT180 | Size: M3 1.8 liter |
| Stand and clamp, or standard tripod for camera | |||
| 250mL beaker | |||
| Tracking software | We use Ethovision XT 13 from Noldus Information Technology | ||
| Buspirone chloride | Sigma-Aldrich | B7148 | |
| Randomized trial generator | We use the RANDBETWEEN function in Microsoft Excel |
Odniesienia
- McEwen, B. S. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 840, 33-44 (1998).
- Tovote, P., Fadok, J. P., Lüthi, A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 16 (6), 317-331 (2015).
- Facchinello, N., et al. Nr3c1 null mutant zebrafish are viable and reveal DNA-binding-independent activities of the glucocorticoid receptor. Scientific Reports. 7 (4371), (2017).
- Ziv, L., et al. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Molecular Psychiatry. , (2013).
- Grone, B. P., Maruska, K. P. Divergent evolution of two corticotropin-releasing hormone (CRH) genes in teleost fishes. Frontiers in Neuroscience. , (2015).
- Fuller, P. J., Lim-Tio, S. S., Brennan, F. E. Specificity in mineralocorticoid versus glucocorticoid action. Kidney International. , (2000).
- Zhdanova, I. V. Sleep and its regulation in zebrafish. Reviews in the Neurosciences. 22 (1), 27-36 (2011).
- Patton, E. E., Zon, L. I. The art and design of genetic screens: zebrafish. Nature Reviews Genetics. , (2001).
- Duboué, E. R. E. R., Hong, E., Eldred, K. C. K. C., Halpern, M. E. M. E. Left Habenular Activity Attenuates Fear Responses in Larval Zebrafish. Current Biology. 27 (14), 2154-2162 (2017).
- Facchin, L., Duboue, E. R., Halpern, M. E. Disruption of Epithalamic Left-Right Asymmetry Increases Anxiety in Zebrafish. Journal of Neuroscience. 35 (48), 15847-15859 (2015).
- Øverli, &. #. 2. 1. 6. ;., Sørensen, C., Nilsson, G. E. Behavioral indicators of stress-coping style in rainbow trout: Do males and females react differently to novelty. Physiology and Behavior. , (2006).
- Levin, E. D., Bencan, Z., Cerutti, D. T. Anxiolytic effects of nicotine in zebrafish. Physiology & behavior. 90 (1), 54-58 (2007).
- Bencan, Z., Sledge, D., Levin, E. D. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacology Biochemistry and Behavior. 94 (1), 75-80 (2009).
- Cachat, J., et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nature Protocols. 5 (11), 1786-1799 (2010).
- Mathuru, A. S., et al. Chondroitin fragments are odorants that trigger fear behavior in fish. Current Biology. , (2012).
- Alsop, D., Vijayan, M. The zebrafish stress axis: Molecular fallout from the teleost-specific genome duplication event. General and Comparative Endocrinology. , (2009).
- Evans, A. N., Liu, Y., MacGregor, R., Huang, V., Aguilera, G. Regulation of Hypothalamic Corticotropin-Releasing Hormone Transcription by Elevated Glucocorticoids. Molecular Endocrinology. , (2013).
- Fenoglio, K. A., Brunson, K. L., Avishai-Eliner, S., Chen, Y., Baram, T. Z. Region-specific onset of handling-induced changes in corticotropin- releasing factor and glucocorticoid receptor expression. Endocrinology. , (2004).
- Liposits, Z., et al. Ultrastructural localization of glucocorticoid receptor (GR) in hypothalamic paraventricular neurons synthesizing corticotropin releasing factor (CRF). Histochemistry. , (1987).
- Facchin, L., Duboué, E. R., Halpern, M. E. Disruption of epithalamic left-right asymmetry increases anxiety in Zebrafish. Journal of Neuroscience. 35 (48), (2015).
- Chin, J. S., et al. Convergence on reduced stress behavior in the Mexican blind cavefish. Developmental Biology. , (2018).
- Wong, K., et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behavioural Brain Research. 208 (2), 450-457 (2010).
- Matsunaga, W., Watanabe, E. Habituation of medaka (Oryzias latipes) demonstrated by open-field testing. Behavioural Processes. 85 (2), 142-150 (2010).
- Walker, C. Chapter 3 Haploid Screens and Gamma-Ray Mutagenesis. Methods in Cell Biology. , (1998).
- Rihel, J., et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 327, 348-351 (2010).
- Peal, D. S., Peterson, R. T., Milan, D. Small molecule screening in zebrafish. Journal of Cardiovascular Translational Research. , (2010).
- Murphey, R., Zon, L. Small molecule screening in the zebrafish. Methods. 39 (3), 255-261 (2006).
- Gammans, R. E., et al. Use of buspirone in patients with generalized anxiety disorder and coexisting depressive symptoms. A meta-analysis of eight randomized, controlled studies. Neuropsychobiology. 25 (4), 193-201 (1992).
- Maaswinkel, H., Zhu, L., Weng, W. The immediate and the delayed effects of buspirone on zebrafish (Danio rerio) in an open field test: A 3-D approach. Behavioural Brain Research. , (2012).
- Gebauer, D. L., et al. Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacology Biochemistry and Behavior. , (2011).
- Maximino, C., et al. Fingerprinting of psychoactive drugs in zebrafish anxiety-like behaviors. PLoS ONE. , (2014).
- Horváth, J., Barkóczi, B., Müller, G., Szegedi, V. Anxious and nonanxious mice show similar hippocampal sensory evoked oscillations under urethane anesthesia: Difference in the effect of buspirone. Neural Plasticity. , (2015).
- Costall, B., Kelly, M. E., Naylor, R. J., Onaivi, E. S. Actions of buspirone in a putative model of anxiety in the mouse. Pharm Pharmacol. 40 (7), 494-500 (1988).
- Barros, M., Mello, E. L., Huston, J. P., Tomaz, C. Behavioral effects of buspirone in the marmoset employing a predator confrontation test of fear and anxiety. Pharmacology Biochemistry and Behavior. , (2001).
- Heinen-Kay, J. L., et al. Predicting multifarious behavioural divergence in the wild. Animal Behaviour. 121, 3-10 (2016).
- Thompson, R. R. J., Paul, E. S., Radford, A. N., Purser, J., Mendl, M. Routine handling methods affect behaviour of three-spined sticklebacks in a novel test of anxiety. Behavioural Brain Research. 306, 26-35 (2016).
- Hamilton, T. J., et al. Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Scientific Reports. , (2017).
- York, R. A., Fernald, R. D. The Repeated Evolution of Behavior. Frontiers in Ecology and Evolution. 4, 143 (2017).
- Jakka, N. M., Rao, T. G., Rao, J. V. Locomotor behavioral response of mosquitofish (Gambusia affinis) to subacute mercury stress monitored by video tracking system. Drug and Chemical Toxicology. , (2007).
- Hu, C. K., Brunet, A. The African turquoise killifish: A research organism to study vertebrate aging and diapause. Aging Cell. , (2018).
- Maximino, C., et al. Measuring anxiety in zebrafish: A critical review. Behavioural Brain Research. 214 (2), 157-171 (2010).
- Maximino, C., Marques de Brito, T., Dias, C. A. G., Gouveia, A., Morato, S. Scototaxis as anxiety-like behavior in fish. Nature protocols. 5 (2), 209-216 (2010).
- Godwin, J., Sawyer, S., Perrin, F., Oxendine, S., Kezios, Z. Adapting the Open Field Test to assess anxiety related behavior in zebrafish. Zebrafish Protocols for Neurobehavioral Research. , 181-189 (2012).
- Agetsuma, M., et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nature Neuroscience. 13 (11), 1354-1356 (2010).
- Valente, A., Huang, K. H., Portugues, R., Engert, F. Ontogeny of classical and operant learning behaviors in zebrafish. Learning and Memory. , (2012).
- Baker, M. R., Goodman, A. C., Santo, J. B., Wong, R. Y. Repeatability and reliability of exploratory behavior in proactive and reactive zebrafish, Danio rerio. Scientific Reports. , (2018).
- Friedrich, R. W., Genoud, C., Wanner, A. A. Analyzing the structure and function of neuronal circuits in zebrafish. Frontiers in Neural Circuits. , 7 (2013).
- Friedrich, R. W., Jacobson, G. A., Zhu, P. Circuit Neuroscience in Zebrafish. Current Biology. 20 (8), (2010).
- Marquart, G. D., et al. A 3D Searchable Database of Transgenic Zebrafish Gal4 and Cre Lines for Functional Neuroanatomy Studies. Frontiers in Neural Circuits. , (2015).
- Randlett, O., et al. Whole-brain activity mapping onto a zebrafish brain atlas. Nature Methods. 12 (11), 1039-1046 (2015).
- Gupta, T., et al. Morphometric analysis and neuroanatomical mapping of the zebrafish brain. Methods. 1046 (18), 30011-30012 (2018).
- Marquart, G. D., et al. High-precision registration between zebrafish brain atlases using symmetric diffeomorphic normalization. GigaScience. , (2017).
- Ronneberger, O., et al. ViBE-Z: A framework for 3D virtual colocalization analysis in zebrafish larval brains. Nature Methods. , (2012).
- Subedi, A., et al. Adoption of the Q transcriptional regulatory system for zebrafish transgenesis. Methods. 66 (3), 433-440 (2014).
- Scheer, N., Campos-Ortega, J. A. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mechanisms of Development. 80 (2), 153-158 (1999).
- Chatterjee, D., Tran, S., Shams, S., Gerlai, R. A Simple Method for Immunohistochemical Staining of Zebrafish Brain Sections for c-fos Protein Expression. Zebrafish. , (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone