Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
In vitro Time-lapse Live-Cell Imaging to Explore Cell Migration toward the Organ of Corti
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
In this study, we present a real-time imaging method using confocal microscopy to observe cells moving toward damaged tissue by ex vivo incubation with the cochlear epithelium containing the organ of Corti.
Streszczenie
To study the effects of mesenchymal stem cells (MSCs) on cell regeneration and treatment, this method tracks MSC migration and morphological changes after co-culture with cochlear epithelium. The organ of Corti was immobilized on a plastic coverslip by pressing a portion of the Reissner's membrane generated during the dissection. MSCs confined by a glass cylinder migrated toward cochlear epithelium when the cylinder was removed. Their predominant localization was observed in the modiolus of the organ of Corti, aligned in a direction similarly to that of the nerve fibers. However, some MSCs were localized in the limbus area and showed a horizontally elongated shape. In addition, migration into the hair cell area was increased, and the morphology of the MSCs changed to various forms after kanamycin treatment. In conclusion, the results of this study indicate that the coculture of MSCs with cochlear epithelium will be useful for the development of therapeutics via cell transplantation and for studies of cell regeneration that can examine various conditions and factors.
Wprowadzenie
Hearing loss can occur congenitally or can be caused progressively by several factors, including aging, drugs, and noise. Hearing loss is often difficult to treat because it is very challenging to restore impaired function once the hair cells responsible for hearing are damaged1. According to the World Health Organization, 461 million people worldwide are estimated to have hearing loss, which accounts for 6.1% of the world's population. Of those with hearing loss, 93% are adults, and 7% are children.
A number of approaches have been attempted to treat hearing loss; notably, a regeneration approach using MSCs has emerged as a promising treatment. When tissue is damaged, MSCs are naturally released into the circulatory system and migrate to the injury site where they secrete various molecules to form a microenvironment that promotes regeneration2. Hence, it is important to develop a method to treat damaged tissues through the migration of externally implanted MSCs to target organs and their subsequent secretion of molecules that cause potent immune regulation, angiogenesis, and anti-apoptosis to enhance the restoration of damaged cell function3,4,5.
The homing process in which MSCs migrate to damaged tissues may be the most important obstacle to overcome. MSCs have a systemic homing mechanism with sequential steps of tethering/rolling, activation, arrest, transmigration/diapedesis, and migration6,7,8. Currently, efforts are underway to identify ways to improve these steps. Various strategies, including genetic modification, cell surface engineering, in vitro priming, and magnetic guidance, have been tested6,7. In addition, several attempts have been made to promote the protection and regeneration of auditory hair cells by homing MSCs to the site of damaged cochlea. However, tracking MSCs in vivo is time-consuming and labor-intensive and requires highly specialized skills9.
To solve this problem, a method was developed to observe the homing of MSCs in the cochlea through time-lapse confocal microscopy that photographs the migration of cells over several hours (Figure 1). It was developed in the early 20th century and has recently become a powerful tool for studying migration of specific cells.
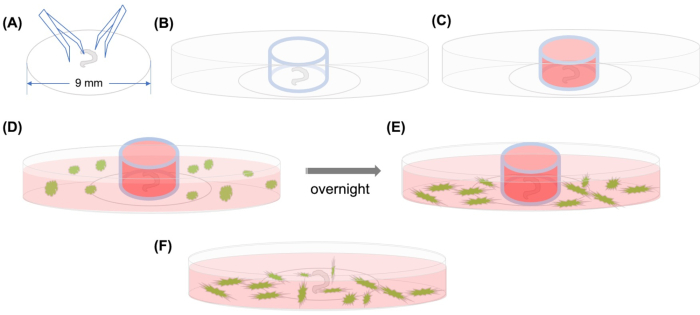
Figure 1: Graphical abstract. (A) After the dissected organ of Corti is adhered on a plastic coverslip using forceps, the coverslip is placed on a 35 mm glass-bottomed confocal microscopic dish, and (B) the glass cylinder is positioned. (C) After filling the inside of the glass cylinder with medium, (D) GFP-labeled MSCs with medium are added carefully outside the cylinder. (E) After incubation overnight, (F) the glass cylinder is removed, and images are taken with a confocal microscope. Abbreviations: GFP = green fluorescent protein; MSCs = mesenchymal stem cells. Please click here to view a larger version of this figure.
Protokół
All research protocols involving ICR mice were approved by the Institutional Animal Care and Use Committee (IACUC) of Yonsei University at Wonju College of Medicine. Experiments were performed according to the Code of Ethics of the World Medical Association. In this protocol, pregnant ICR mice were kept in a 12/12 h light/dark cycle with free access to food and water.
1. Cochleae dissection
- Sterilize the laminar flow tissue culture hood by turning on the ultraviolet light for ~30 min, and spray all surfaces with 70% ethanol prior to use. Allow the surfaces to dry.
- Place dissection instruments in 70% ethanol for 10 min, and dry before using.
- Use an operating blade to decapitate postnatal 3-4 day-old mice (Figure 2A).
- Place the skull under a stereomicroscope in the laminar flow hood, and soak the tissue in 70% ethanol.
- Quickly soak the tissue in tissue dissection solution (1x Hank's Balanced Salt Solution, 1 mM HEPES) to remove the ethanol.
- Cut the centerline of the skull with a surgical blade (Figure 2B,C).
- Expose the skull by pulling down the skin anteriorly and cutting the external auditory canal of the ear (Figure 2D).
- Cut from the anterior to the posterior part of the cranium across the eye line (Figure 2E).
- Open the skull and remove the forebrain, cerebellum, and brainstem with blunt forceps (Figure 2F,G).
- Using micro forceps, separate the cochlea from the temporal bone (Figure 2H).
- Transfer the cochleae to a Petri dish containing tissue dissection solution.
- Carefully dissect all of the cochlear otic capsule, leaving only the internal cochlear soft tissue (Figure 2I,J).
- Hold the modiolus of the cochleae with forceps and the cochlear duct with another pair of forceps, and slowly separate the two tissues (Figure 2K).
- Remove the stria vascularis and tectorial membrane by gently peeling them away (Figure 2L,M).
- Place a sterilized plastic coverslip in new tissue dissection solution, and then place the organ of Corti on a coverslip of 9 mm diameter, making sure that the basilar membrane faces downward (Figure 2N-P).
- Immobilize the tissue by pressing the Reissner's membrane and the remaining modiolus tissue onto the coverslip with forceps (Figure 2N-P).
- Transfer the coverslip with the embedded tissue to the center of a confocal dish of 35 mm diameter.
- Place the glass cloning cylinder on the dish, with the cochlear explant positioned in the center of the dish, and add 100 µL of explant culture medium (DMEM/F12, 10% fetal bovine serum (FBS), 1% N2 supplement, ampicillin (10 µg/mL))10 inside the cylinder (Figure 2Q).
- Plate 5 × 103 cells of mouse bone marrow-derived green fluorescent protein (GFP)-tagged MSCs in 2 mL of culture medium (45% DMEM + 45% DMEM/F12, 10% FBS, 1% N2 supplement, 10 µg/mL of ampicillin) outside the glass cylinder (Figure 2R).
- When the MSCs are 80-90% confluent, passage them by detaching them with trypsin-ethylenediamine tetraacetic acid.
- Carefully transfer the confocal dish to a humidified incubator and incubate overnight at 37 °C in a 5% CO2 atmosphere.
- Aspirate all medium inside and outside the cylinder, and then remove the glass cylinder from the confocal dish.
- Add 2 mL of fresh culture medium to the confocal dish, and incubate the tissue culture dish in a humidified incubator until ready for analysis.

Figure 2. Dissection of a mouse cochlea and coculture of the organ of Corti and MSCs. (A) Decapitation of mouse, (B) and (C) midline sagittal dissection of the head, (D) and (E) coronal dissection of the brain, (F) and (G) removal of the brain and temporal bone, (H) the cochlea, (I) removal of the bony cochlear wall, (J) isolation of the cochlea, (K) separation of the cochlear duct from the modiolus, (L) separation of stria vascularis (SV) and spiral ligament (SL) from the organ of Corti, (M) removal of the tectorial membrane, (N-P) fixation of the cochlea on a plastic cover slip, (Q) location of coverslip and glass cylinder in the confocal dish, (R) inoculation of MSCs. White scale bar (A-E) = 1 cm; orange (F, G, P) and yellow scale bar (H,I) = 1 mm; green scale bar (J-O) = 0.5 mm. Please click here to view a larger version of this figure.
2. Time-lapse imaging
- For the experiments presented here, use a confocal microscopy system with a stage top incubator system.
- Turn on the confocal microscope, fluorescent light, and computer.
- Set the conditions of the stage-top incubator placed on the stage of the confocal microscope to 37 °C and 5% CO2 atmosphere.
- Place the sample dish on the dish fixing vessel, cover with the dish fixing lid, and close the chamber with the top heater lid.
- Adjust the zoom and focus to localize the organ of Corti and MSCs in the field of view.
- Open the image processing software. Under the locate option, select a 20x Plan-Apochromat objective (numerical aperture 0.8) and a 0.5x crop area.
- Under Acquisition, click on smart setup and select EGFP.
- Open the channel tab under Acquisition, and set the laser power to 0.2%, the pinhole to 44 µm, the master gain to 750 V, and the digital gain to 1.0.
- Click on ESID under imaging setup, and set the ESID gain to 4 and the digital gain to 7.5.
- Click on Tiles and stake to produce 210 tiles.
- Open Focus strategy and select the focus mode.
- Under time series, set the duration to 24 h and the interval to 10 min.
- Under Acquisition, set the frame size to 512 x 512 pixels, scan speed to 8, the direction to bidirectional, the averaging to 4x, and the bits per pixel to 16.
- Click on start experiment to begin the experiment.
3. Image file modification
- Under processing, click on stitching, and set the minimal overlay to 5% and the maximal shift to 10%.
- Click on movie export, set uncompressed, and set the speed to 7.5.
4. Immunostaining
- Aspirate the medium carefully, and wash the sample twice with phosphate-buffered saline (PBS) for 5 min.
- Fix the sample with 4% formalin in PBS for 15 min, and wash the sample 3 times with PBS for 5 min.
- Permeabilize the sample in 0.1% Triton X-100 in PBS for 10 min, and wash 3 times with PBS for 5 min.
- Add 250 µL of phalloidin-iFluor 647 reagent (1:1000 dilution in PBS), and incubate the sample for 1 h at room temperature on the shaker.
- Wash the sample 3 times with PBS for 5 min.
- Transfer the coverslip onto the glass slide, and add 2 drops of mounting solution.
- Place a coverslip on the slide gently.
- Seal the coverslip with clear nail polish and store at 4 °C in the dark until cells are observed.
- Image the slide using a confocal microscope with an appropriate filter at excitation/emission (Ex/Em)=650/665 nm for phalloidin and at Ex/Em=488/507 nm for EGFP.
Wyniki
In vitro migration of MSCs in three-dimensional mode has been assessed by a Transwell system or by the traditional wound healing method to observe migration in two-dimensional (2D) mode11. The organ of Corti is a complex structure composed of various cells such as Boettcher cells, Claudius cells, Deiters cells, pillar cells, Hensen's cells, outer hair cells, inner hair cells, nerve fibers, basilar membrane, and reticular lamina12. When M...
Dyskusje
Transplantation of MSCs into damaged sites to promote the regeneration of damaged cells has been extensively studied, and the therapeutic effect is evident.The transplantation and subsequent differentiation of MSCs have been reported to restore hearing in rats with hearing loss induced by 3-nitropropionic acid13. Although Lee et al. applied MSCs to human beings trans-venously, they did not achieve any significant improvement in hearing14. Until recently, nearly 12 expe...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by research grants (NRF-2018-R1D1A1B07050175, HURF-2017-66) from the National Research Foundation (NRF) of Korea and Hallym University Research Fund.
Materiały
| Name | Company | Catalog Number | Comments |
| 10X PBS Buffer | GenDEPOT | P2100-104 | |
| 4% Formalin | T&I | BPP-9004 | |
| Ampicillin | sigma | A5354-10ml | |
| BSA | sigma | A4503-100G | |
| confocal dish | SPL | 200350 | |
| confocal microscope | ZEISS | LSM800 | |
| coverslip | SPL | 20009 | |
| DMEM/F12 | Gibco | 10565-018 | |
| Fetal Bovine Serum | Thermo Fisher scientific | 16140071 | |
| Fluorsheild with DAPI | sigma | F6057 | |
| Forcep | Dumont | 0508-L5-P0 | |
| HBSS | Thermo Fisher scientific | 14065056 | |
| HEPES | Thermo Fisher scientific | 15630080 | |
| N2 supplement | Gibco | 17502-048 | |
| Phalloidin-iFluor 647 Reagent | abcam | ab176759 | |
| Stage Top Incubator | TOKAI HIT | WELSX | |
| Strain C57BL/6 mouse messenchymal stem cells with GFP | cyagen | MUBMX-01101 | |
| Triton X-100 | sigma | T8787 |
Odniesienia
- Brown, C. S., Emmett, S. D., Robler, S. K., Tucci, D. L. Global hearing loss prevention. Otolaryngologic Clinics of North America. 51 (3), 575-592 (2018).
- Chamberlain, G., Fox, J., Ashton, B., Middleton, J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 25 (11), 2739-2749 (2007).
- Fu, X., et al. Mesenchymal stem cell migration and tissue repair. Cells. 8 (8), (2019).
- Uder, C., Brückner, S., Winkler, S., Tautenhahn, H. M., Christ, B. Mammalian MSC from selected species: Features and applications. Cytometry A. 93 (1), 32-49 (2018).
- Rojewski, M. T., et al. Translation of a standardized manufacturing protocol for mesenchymal stromal cells: A systematic comparison of validation and manufacturing data. Cytotherapy. 21 (4), 468-482 (2019).
- Ullah, M., Liu, D. D., Thakor, A. S. Mesenchymal stromal cell homing: Mechanisms and strategies for improvement. iScience. 15, 421-438 (2019).
- Ahn, Y. J., et al. Strategies to enhance efficacy of SPION-labeled stem cell homing by magnetic attraction: a systemic review with meta-analysis. International Journal of Nanomedicine. 14, 4849-4866 (2019).
- Alon, R., Ley, K. Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Current Opinion in Cell Biology. 20 (5), 525-532 (2008).
- Sykova, E., Jendelova, P. In vivo tracking of stem cells in brain and spinal cord injury. Progress in Brain Research. 161, 367-383 (2007).
- Landegger, L. D., Dilwali, S., Stankovic, K. M. Neonatal murine cochlear explant technique as an in vitro screening tool in hearing research. Journal of Visualized Experiments. (124), e55704 (2017).
- Pijuan, J., et al. In vitro cell migration, invasion, and adhesion assays: From cell imaging to data analysis. Frontiers in Cell and Developmental Biology. 7, 107 (2019).
- Rask-Andersen, H., et al. Human cochlea: anatomical characteristics and their relevance for cochlear implantation. The Anatomical Record. 295 (11), 1791-1811 (2012).
- Kamiya, K., et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. The American Journal of Pathology. 171 (1), 214-226 (2007).
- Lee, H. S., Kim, W. J., Gong, J. S., Park, K. H. Clinical safety and efficacy of autologous bone marrow-derived mesenchymal stem cell transplantation in sensorineural hearing loss patients. Journal of Audiology and Otology. 22 (2), 105-109 (2018).
- Vanden Berg-Foels, W. S. In situ tissue regeneration: chemoattractants for endogenous stem cell recruitment. Tissue Engineering Part B: Reviews. 20 (1), 28-39 (2014).
- Parker, M., Brugeaud, A., Edge, A. S. Primary culture and plasmid electroporation of the murine organ of Corti. Journal of Visualized Experiments. (36), e1685 (2010).
- Ogier, J. M., Burt, R. A., Drury, H. R., Lim, R., Nayagam, B. A. Organotypic culture of neonatal murine inner ear explants. Frontiers in Cellular Neuroscience. 13, 170 (2019).
- Oshima, K., et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 141 (4), 704-716 (2010).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone