Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Preparation and Structural Evaluation of Epithelial Cell Monolayers in a Physiologically Sized Microfluidic Culture Device
W tym Artykule
Podsumowanie
The presented protocol describes the development and use of a phalloidin-based filamentous-actin staining technique with confocal laser scanning microscopy (CLSM) to visualize adherent cell layer structure in microfluidic dynamic-culture channels and traditional fixed-well static-culture chambers. This approach aids in evaluating cell layer confluency, monolayer formation, and layer-thickness uniformity.
Streszczenie
In vitro microfluidic experimentation holds great potential to reveal many insights into the microphysiological phenomena occurring in conditions such as acute respiratory distress syndrome (ARDS) and ventilator-induced lung injury (VILI). However, studies in microfluidic channels with dimensions physiologically relevant to the terminal bronchioles of the human lung currently face several challenges, especially due to difficulties in establishing appropriate cell culture conditions, including media flow rates, within a given culture environment. The presented protocol describes an image-based approach to evaluate the structure of NCI-H441 human lung epithelial cells cultured in an oxygen-impermeable microfluidic channel with dimensions physiologically relevant to the terminal bronchioles of the human lung. Using phalloidin-based filamentous-actin staining, the cytoskeletal structures of the cells are revealed by confocal laser scanning microscopy, allowing for the visualization of individual as well as layered cells. Subsequent quantification determines whether the cell culture conditions being employed are producing uniform monolayers suitable for further experimentation. The protocol describes cell culture and layer evaluation methods in microfluidic channels and traditional fixed-well environments. This includes channel construction, cell culture and requisite conditions, fixation, permeabilization and staining, confocal microscopic imaging, image processing, and data analysis.
Wprowadzenie
Acute respiratory distress syndrome (ARDS) is an acute condition arising from insult to and propagation of injury in the lung parenchyma, resulting in pulmonary edema of the alveoli, inadequate gas exchange, and subsequent hypoxemia1. This initiates a cycle of pro-inflammatory cytokine release, neutrophil recruitment, toxic mediator release, and tissue damage, which itself incurs a further inflammatory response2. Additionally, pulmonary surfactant, which stabilizes the airways and prevents damage caused by repetitive recruitment/derecruitment (R/D), may be inactivated or otherwise rendered dysfunctional by the chemical processes occurring during ARDS, resulting in further stress and injury to the surrounding parenchyma3. If sufficient damage is sustained, mechanical ventilation may be necessary to ensure adequate systemic oxygenation4. However, mechanical ventilation imposes its own challenges and traumas, including the possibility of ventilator-induced lung injury (VILI), characterized as injury to the lung parenchyma caused by the mechanical stresses imposed during overinflation (volutrauma) and/or the R/D of the air-liquid interface in the fluid-occluded airway (atelectrauma)5. The pressure gradient experienced by epithelial cells exposed to an air-liquid interface (as in a fluid-occluded bronchiole) in the atelectrauma model can result in a permeability-originated obstructive response (POOR), leading to a POOR-get-POORer virtuous cycle of injury6,7,8.
In vitro experimentation can provide micro-scale insights into these phenomena, but current studies in microfluidic channel environments with physiologically relevant dimensions face several challenges9. For one, optimizing cell culture conditions poses a significant barrier to entry for cell culture research in microfluidic environments, as there exists a narrow intersection within which media flow parameters, culture duration, and other culture conditions permit optimal cell layer formation. This includes the diffusion limitations imposed by the oxygen-impermeable nature of the microfluidic culture channel enclosure. This necessitates careful consideration of media flow parameters, as low flow rates can deprive cells of oxygen, especially those farthest from the inlet; on the other hand, high flow rates can push cells out of the culture channel or result in improper or uneven layer development. Diffusion limitations may be addressed by using oxygen-permeable materials such as polydimethylsiloxane (PDMS) in an air-liquid interface (ALI) culture apparatus; however, many conventional microfluidic culture channels, such as those of the electric cell-substrate impedance sensing (ECIS) system, are inherently oxygen-impermeable, given the nature of the manufactured enclosure10. This protocol aims to provide a technique for analyzing cell layers cultured in an oxygen-impermeable enclosure.
When comparing the viability of culture conditions, observations of specific layer characteristics, such as the presence of a monolayer, surface topology, confluency, and layer-thickness uniformity, are necessary to determine whether the cell layer produced by a particular set of culture conditions meets the desired specifications and are indeed relevant to the experimental design. A limited evaluation may be performed by methods such as ECIS, which utilizes measurements of electric potential (voltage) created by resistance to high-frequency alternating current (AC) (impedance) imposed by electrically-insulating membranes of cells cultured on gold electrodes within the flow array. By modulating the frequency of AC applied to cells, specific frequency-dependent cellular properties of the cells and cell layers such as surface adherence strength, tight-junction formation, and cell proliferation or confluency may be targeted and examined11. However, these indirect forms of measurements are somewhat difficult to interpret at the onset of an experiment, and may not quantify all relevant aspects of the cell layer. Simply observing the cell layer under a phase-contrast microscope may reveal the nature of certain qualities such as confluency; however, many relevant characteristics such as the presence of a monolayer and layer-thickness uniformity require a three-dimensional (3D) evaluation that is not possible with brightfield, phase-contrast, or fluorescent microscopic imaging12.
The objective of this study was to develop a filamentous-actin staining technique to allow for imaging-based verification of a monolayer and the evaluation of cell layer uniformity using confocal laser scanning microscopy (CLSM). Filamentous-actin (F-actin) was deemed an appropriate target for the fluorophore conjugate, due in part to the way that F-actin tightly follows the cell membrane, allowing for a visual approximation of the entire cell volume13. Another important benefit of targeting F-actin is the manner in which staining of F-actin visually elucidates cytoskeletal disruptions or alterations imposed by the stresses and strains experienced by the cells. Crosslinking fixation with methanol-free formaldehyde was used to preserve the morphology of the cells and the cell layer, as dehydrating fixatives such as methanol tend to flatten cells, grossly distorting the cell layer and altering its properties14,15.
To determine the ability of the layer evaluation technique to mitigate these challenges, cells were cultured in traditional eight-well culture chambers as well as in microfluidic channels to evaluate the differences, if any, in the cell layers that were produced. For fixed culture wells, eight-well chambered coverglass units were used. For microfluidic culture, flow arrays (channel length 50 mm, width 5 mm, depth 0.6 mm) were optimized to culture immortalized human lung epithelial (NCI-H441) cells in an environment with dimensions physiologically relevant to the terminal bronchioles present in the respiratory zone of the human lung16. While this protocol was developed with the culture environment of ECIS flow arrays in mind, it may apply to any oxygen-impermeable dynamic-culture environment for which evaluation of cultured cell layer characteristics or culture conditions is necessary.
Protokół
The NCI-H441 human epithelial lung cell line was used for the present study (see Table of Materials).
1. Cell culture in the microfluidic channel
- Fabricate the microfluidic channel and perform the pre-treatment following the steps below.
- Obtain a single-channel flow array (see Table of Materials) and separate the upper portion from the polycarbonate base plate.
- Obtain a #1.5 rectangular coverglass (thickness 0.17 mm) with dimensions of 60 mm x 22 mm. Clean surfaces of the coverglass in an ultrasonic bath and treat one side with a 0.1 mg/mL solution of Poly-D-Lysine at room temperature for 5 min before drying at 60 °C for 30 min.
- Affix a 0.13 mm thick double-sided adhesive (see Table of Materials), laser-cut to accommodate the dimensions of the flow array top and the flow channel (50 mm length, 5 mm width), to the flow array top, taking care to precisely align the channel cut-outs17.
- Affix a 0.1 mm thick mylar spacer (see Table of Materials), laser-cut to accommodate the dimensions of the flow array top and the flow channel, to the adhesive strip, taking care to precisely align the channel cut-outs.
- Repeat steps 1.1.3 and 1.1.4 until desired channel height is achieved (for example, for a channel height of 0.6 mm, use two spacers and three adhesive strips).
- Affix a rectangular coverglass to the bottom-most adhesive strip with the Poly-D-Lysine-treated side facing the adhesive. After assembly is complete, as indicated in Figure 1, apply firm and equal pressure to the top and bottom of construction and hold for 1 min.
NOTE: Construction of the channel enclosure, including coverglass, adhesives, spacers, and flow array top, is now complete. - Rinse the channel with de-ionized water using a syringe, simultaneously checking for leaks.
- Sterilize channel enclosure in an ultraviolet (UV) sterilizer for 30 min18.
- Using sterile technique, treat the channel with 2.0 µg/mL human fibronectin (see Table of Materials) in phosphate buffered saline (PBS) and incubate for at least 30 min at 37 °C19.
- Perform cell culture in the microfluidic channel following the steps below.
- In a sterile laminar flow hood, use a micropipette to transfer an even suspension of NCI-H441 cells in RPMI 1640 medium with 10% fetal bovine serum (FBS) (see Table of Materials) to seed two microfluidic channels, each with cells at a surface density of 150,000 cells/cm2.
- For 50 mm x 5 mm x 0.6 mm channels, use 0.25 mL of a 2.5 x 106 cells/mL suspension to fill each channel, as well as a portion of the ports. Verify that cells have been distributed evenly within the channels using a brightfield microscope.
- Culture the two channels for 24 h and 48 h respectively at 37 °C with 5% CO2 using a programmable syringe pump (see Table of Materials), drawing spent media out from the channel and fresh media into the channel from a sterile media reservoir attached to channel inlet comprised of a cut-open 20 mL syringe covered with paraffin film.
- After a 10 min waiting period following cell seeding, introduce and pump fresh media from the reservoir through the channel at a variable flow rate beginning at 0.2 µL/min and ramping up to 10 µL/min for 4 h, maintaining at that rate thereafter20.
NOTE: This variable flow rate provides culture conditions that allow cells to (1) gravitationally settle to the culture surface, (2) adhere to the culture surface, and (3) form a confluent monolayer.
- After a 10 min waiting period following cell seeding, introduce and pump fresh media from the reservoir through the channel at a variable flow rate beginning at 0.2 µL/min and ramping up to 10 µL/min for 4 h, maintaining at that rate thereafter20.
- In a sterile laminar flow hood, use a micropipette to transfer an even suspension of NCI-H441 cells in RPMI 1640 medium with 10% fetal bovine serum (FBS) (see Table of Materials) to seed two microfluidic channels, each with cells at a surface density of 150,000 cells/cm2.
- Perform cell fixation inside the microfluidic channels using formaldehyde solution.
CAUTION: Formaldehyde is toxic and must be handled in an appropriate chemical fume hood21.- In a chemical fume hood, prepare formaldehyde solutions by using 4% formaldehyde in PBS (methanol-free) (see Table of Materials) to create two 4 mL portions, diluting the first to a concentration of 1% formaldehyde and the second to 2% formaldehyde using Dulbecco's phosphate buffered saline (DPBS; with Ca2+ and Mg2+) as the diluent. Transfer formaldehyde solutions to separate 5 mL syringes and label accordingly. Draw up 20 mL of DPBS into a separate 20 mL syringe.
- Remove microfluidic channels from the culture apparatus and place them into the chemical fume hood.
- Assemble the fixation and staining apparatus.
- Attach a 10 cm segment of transfer tubing to the side port of a three-way stopcock via a male Luer lock to hose barb adapter (see Table of Materials), then connect the stopcock to the inlet port of the flow array.
- Next, attach another 10 cm segment of transfer tubing to the outlet port of the flow array using the same type of hose barb adapter.
- Finally, secure the free ends of both transfer tubes in a chemical and biohazard-appropriate waste container, such as a labeled empty 50 mL conical centrifuge tube.
- Turn the stopcock to block off the flow array inlet port and flush the waste line with DPBS. Then, turn the stopcock to block off the waste line and slowly wash cells with 2 mL of DPBS. Repeat the flushing step using the new solution every time. A new solution (or concentration of solution) is introduced to the channel.
- Slowly push 2 mL of 1% fixative solution through the channel and then allow to sit for 5 min22.
- Slowly push 2 mL of 2% fixative solution through the channel and then allow to sit for 15 min.
- Wash cells by slowly introducing 2 mL of fresh DPBS to the channel in three separate instances (5 min each).
- Complete steps 1.3.3-1.3.7 for both microfluidic channels in parallel.
- Stain, permeabilize, and add mounting media to the cells in the microfluidic channel.
- Prepare 0.1% saponin solution by adding 1 mg of saponin (see Table of Materials) per mL of DPBS to produce 4 mL of solution and gently vortex to mix23. Draw up 8 mL of DPBS into a 20 mL syringe.
- Add an F-actin-staining phalloidin reagent and a nucleus-staining Hoechst reagent (see Table of Materials) to the 0.1% saponin solution, at two drops (0.1 mL) of each reagent per mL of saponin solution. Keep prepared staining/permeabilizing solution away from light by covering with aluminum foil24.
- Flush the line with a small amount of staining/permeabilizing solution (as described in step 1.3.4), then introduce 2 mL of the solution to the microfluidic channel and cover the channel with aluminum foil before allowing it to sit at room temperature for 30 min.
- Flush out the staining/permeabilizing solution twice with 2 mL of DPBS for 5 mins per flush.
- For better image quality, add a suitable mounting media (with an index of refraction closely matching the microscope objective oil and cover glass, see Table of Materials) into the channel.
- Using a micropipette, introduce a minimal amount of a soft-set antifade mountant to each port of the microfluidic channel, ensuring that the bottom surface is completely covered and no bubbles are trapped within the desired imaging area25. Seal the ends of the channel and verify cell layer integrity by observing under a brightfield microscope.
- Complete steps 1.4.3-1.4.5 for both microfluidic channels in parallel.
NOTE: Image cells as soon as possible after staining for maximum image quality. If photobleaching occurs or long-term storage is desired, other mounting media with antifade or sample-preserving properties may be used. Note that hard-curing mounting media will distort the 3D structure of the cells and, by extension, the cell layer; for this reason, soft-setting mounting media is preferable26.
- Image cells in the microfluidic channel following the steps below.
- Adjust the confocal microscope (see Table of Materials) settings, including laser power, gain, offset, and scan parameters such as scan speed, scan area, scan format, resolution, and pinhole diameter27.
- Test imaging location by taking reference scans as well as Z-stacks until desired image parameters and conditions have been met. Dial-in parameters at sequentially higher-magnification objectives until the 40x oil-immersion objective has been reached and optimized28.
- Using the flow array base plate as a reference, construct Z-stacks at five locations, at the would-be location of the first electrode on the inlet side, halfway between the center and the previous location, the center, halfway between the center and the location of the last electrode (on the outlet side), and at the last electrode, as indicated in Figure 2.
- Perform image processing and data analysis.
- Export XZ and YZ cross-sections using the confocal microscope software package (see Table of Materials).
- Using an image-processing software (see Table of Materials) with edge-detection capabilities (thresholding value 15.0), measure the total image area in pixels by using the Magic Wand tool outside of the image, then the area excluding the total cross-sectional area of the cell layer in pixels by using the Magic Wand tool in the portion of the image exterior to the cell layer29.
- Using data-processing software (see Table of Materials), subtract the exterior area pixel value from the total area pixel value to find the cross-sectional area pixel value.
- Convert cross-sectional area pixel values to µm2 values by multiplying pixel values by the square of the µm/pixel value, as indicated in the microscope software for the particular image (0.31 µm/pixel for Z-stacks taken in 1024p resolution with no zoom on a 40x oil-immersion lens).
- Calculate means and standard deviations of data and graph results.
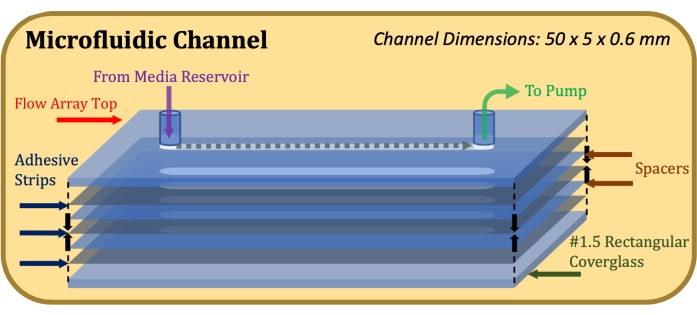
Figure 1: Exploded-view schematic of the microfluidic channel construction. The top element is the top portion of the flow array, thin grey elements are adhesive strips, thin blue elements are mylar spacers, and the bottom element is the rectangular coverglass. Please click here to view a larger version of this figure.
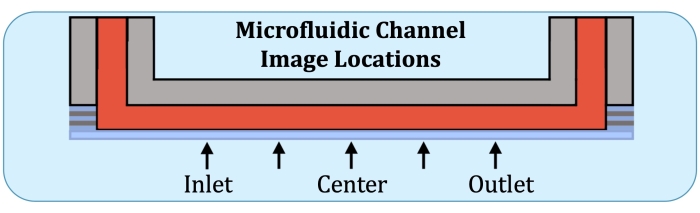
Figure 2: Five imaging locations along the consistently layer-producing region of the microfluidic culture channel. Imaging locations are as follows: inlet-side, near where the first electrode would be on the intact flow array; halfway between inlet-side location and the center of the channel; center of the channel; halfway between the center and the outlet-side location, and outlet-side, near where the last electrode would be on the intact flow array. Please click here to view a larger version of this figure.
2. Cell culture in the eight-well chambered coverglass
- Perform pre-treatment of the eight-well chambered coverglass.
- Obtain a sterile eight-well chambered coverglass manufactured with a #1.5 coverglass and cell adherence-increasing surface treatment (Figure 3, see Table of Materials).
- Using sterile technique, treat the surface of culture wells with 2.0 µg/mL human fibronectin in PBS and incubate for at least 30 min at 37 °C19.
- Perform cell culture in the chambered coverglass following the steps below.
- In a sterile laminar flow hood, transfer 0.5 mL portions of solutions of NCI-H441 cells evenly suspended in RPMI 1640 medium with 10% FBS at volumetric densities of 81,000, 162,000, and 324,000 cells/mL to seed the culture wells at surface densities of 45,000, 90,000, and 180,000 cells/cm2, respectively. Verify that cells have been evenly distributed within the wells using a brightfield microscope.
- Culture cells for 24 h, 48 h, and 96 h at 37 °C with 5% CO2, replacing media daily.
- Perform formaldehyde fixation in the eight-well chambered coverglass.
CAUTION: Formaldehyde is toxic and must be handled in an appropriate chemical fume hood21.- In a chemical fume hood, prepare formaldehyde solutions by making two portions of 4% formaldehyde in PBS (methanol-free), diluting the first to a concentration of 1% formaldehyde and the other to 2% formaldehyde using Dulbecco's phosphate buffered saline (DPBS; with Ca2+ and Mg2+) as the diluent.
- Remove the eight-well culture chambered coverglass from the incubator and place it into the chemical fume hood.
- Gently wash cells with 0.5 mL of DPBS using a micropipette by slowly introducing liquid along the upper portion of the corner of each well.
- Remove existing liquid in each well by slowly extracting it from the corner of the wells using a micropipette. Using the liquid-introduction method (step 2.3.3), introduce 0.5 mL of 1% fixative solution into each well and allow it to sit for 5 min22.
- Remove existing liquid in each well using the liquid-extraction method mentioned in step 2.3.4. Using the liquid-introduction method mentioned in step 2.3.3, introduce 0.5 mL of 2% fixative solution into each well and allow it to sit for 15 min.
- Using the liquid introduction and extraction methods (steps 2.3.3 and 2.3.4), wash cells by introducing and removing 0.5 mL of fresh DPBS in each well in three separate instances for 5 min each.
- Perform staining, permeabilization, and mounting media addition in the eight-well chambered coverglass.
- Prepare 0.1% saponin solution by adding 1 mg of saponin per mL of DPBS and gently vortex to mix23.
- To the 0.1% saponin solution, add two drops (0.1 mL) each of an F-actin-staining phalloidin reagent and a nucleus-staining Hoechst reagent per mL of saponin solution. Keep the prepared solution away from light by covering with aluminum foil24.
- Introduce 0.2 mL of staining/permeabilizing solution to each well and cover the chambered coverglass with aluminum foil before allowing it to sit at room temperature for 30 min.
- Flush out staining/permeabilizing solution twice with 0.5 mL of DPBS.
- For better image quality, add a suitable mounting media (with an index of refraction closely matching the microscope objective oil and cover glass) into the wells.
- Using a micropipette, introduce a minimal amount of a soft-set antifade mountant to each well, ensuring that the bottom surface is completely covered and no bubbles are trapped within the desired imaging area25. Verify cell layer integrity by observing under a brightfield microscope.
NOTE: Image cells as soon as possible after staining for maximum image quality. If photobleaching occurs or long-term storage is desired, other mounting media with antifade or sample-preserving properties may be used. Note that hard-curing mounting media will distort the 3D structure of the cells and, by extension, the cell layer, so soft-setting mounting media is preferable26.
- Using a micropipette, introduce a minimal amount of a soft-set antifade mountant to each well, ensuring that the bottom surface is completely covered and no bubbles are trapped within the desired imaging area25. Verify cell layer integrity by observing under a brightfield microscope.
- Perform imaging in the eight-well chambered coverglass.
- Adjust the confocal microscope settings, including laser power, gain, offset, and scan parameters such as scan speed, scan area, scan format, resolution, and pinhole diameter27.
- Test imaging location by taking reference scans as well as Z-stacks until desired image parameters and conditions have been met. Dial-in parameters at sequentially higher-magnification objectives until the 40x oil-immersion objective has been reached and optimized28.
- Construct Z-stacks of three random locations in each seeding density/culture duration match-up.
- Perform image processing and data analysis.
- Export XZ and YZ cross sections using the confocal microscope software package.
- Using an image-processing software with edge-detection capabilities (thresholding value 15.0), measure the total image area in pixels by using the Magic Wand tool outside of the image, then the area excluding the total cross-sectional area of the cell layer in pixels by using the Magic Wand tool in the portion of the image exterior to the cell layer29.
- Using a data-processing software, subtract the exterior area pixel value from the total area pixel value to find the cross-sectional area pixel value.
- Convert cross-sectional area pixel values to µm2 values by multiplying pixel values by the square of the µm/pixel value as indicated in the microscope software for the particular image (0.31 µm/pixel for Z-stacks taken in 1024p resolution with no zoom on a 40x oil-immersion lens).
- Calculate means and standard deviations and graph results.
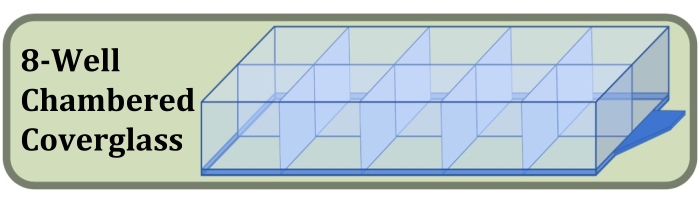
Figure 3: Diagram of the eight-well chambered coverglass used for the fixed-well culture, staining, and imaging experiment comparing the effects of initial cell seeding density and culture duration on the formation of cell layers. Please click here to view a larger version of this figure.
Wyniki
The presented method allows for the visualization of epithelial cell layers cultured in microfluidic culture channels and uses a demonstration in traditional fixed-well cell culture environments as validation. Images acquired will exist on a spectrum of quality, signal intensity, and cellular target specificity. Successful images will demonstrate high contrast, allowing for image analysis and quantification of data for subsequent statistical evaluation. Unsuccessful images will be dim, fuzzy, blurry, or otherwise unusabl...
Dyskusje
The presented protocol describes the culture, crosslinking fixation, staining, permeabilization, and confocal microscopic visualization of NCI-H441 human lung epithelial cells in the dynamic environment of a single-channel microfluidic flow array, as well as in the static environment of a traditional eight-well chambered coverglass. With any microfluidic cell culture protocol, the flow conditions of the cell culture media are of paramount importance, as the high-rate flow has the potential to wash away the cells or inter...
Ujawnienia
The authors declare no conflicts of interest.
Podziękowania
The authors acknowledge Alan Shepardson for designing the cutting pattern for the 3M adhesive and mylar sheet used in microfluidic channel construction and for testing the cell culture media flow rate and syringe pump programming. Funding was supplied by NIH R01 HL0142702, NSF CBET 1706801, and the Newcomb-Tulane College Dean's Grant.
Materiały
| Name | Company | Catalog Number | Comments |
| A1R HD25 Confocal Microscope System | Nikon | A1R HD25 | https://www.microscope.healthcare.nikon. com/products/confocal-microscopes/a1hd25-a1rhd25/specifications |
| ActinGreen 488 ReadyProbes Reagent (AlexaFluor 488 phalloidin) | Invitrogen | R37110 | https://www.thermofisher.com/order/catalog/product/R37110 |
| Adhesive Transfer Tape Double Linered | 3M | 468MP | https://gizmodorks.com/3m-468mp-adhesive-transfer-tape-sheet-5-pack/ |
| Air-Tite HSW Soft-Ject Disposable Syringes | Air-Tite RL5 | 14-817-53 | https://www.fishersci.com/shop/products/air-tite-hsw-soft-ject-disposable-syringes-6/1481753#?keyword=syringe%20leur%20locking%205ml |
| BAISDY 4 mil (0.1 mm) Thick Mylar Sheet | BAISDY | AS022 | https://www.amazon.ca/Stencil-Perfect-Silhouette-Machines-BAISDY/dp/B07RJJ9BNC |
| Branson Ultrasonics M Series Ultrasonic Cleaning Bath | Branson Ultrasonics | 15-336-100 | https://www.fishersci.com/shop/products/m-series-ultrasonic-cleaning-bath/15336100 |
| Corning Fibronectin, Human | Fisher Scientific | CB-40008 | https://www.fishersci.com/shop/products/corning-fibronectin-human-3/CB40008?keyword=true |
| DPBS, calcium, magnesium | Gibco | 14040133 | https://www.thermofisher.com/order/catalog/product/14040133?SID=srch-srp-14040133 |
| ECIS Cultureware Disposable Electrode Arrays 8 x 10 ECIS Flow Array | Applied BioPhysics | 1F8x10E PC | https://www.biophysics.com/cultureware.php#1F8x10E |
| Enterprise Technology Solutions UV Sterilizer Cabinet, White | Enterprise Technology Solutions | 50-211-1163 | https://www.fishersci.com/shop/products/uv-sterilizer-cabinet-white/502111163 |
| Fetal Bovine Serum (FBS) | Gibco | 26140079 | https://www.thermofisher.com/order/catalog/product/26140079 |
| Finnpipette F2 Variable Volume Pipettes | Thermo Scientific | 4642090 | https://www.thermofisher.com/order/catalog/product/4642090 |
| Fisherbrand 50mL Easy Reader Plastic Centrifuge Tubes | Fisher Scientific | 06-443-21 | https://www.fishersci.com/shop/products/fisherbrand-higher-speed-easy-reader-plastic-centrifuge-tubes-8/p-193269 |
| Fisherbrand Cover Glasses: Rectangles (#1.5) | Fisher Scientific | 12-544-GP | https://www.fishersci.com/shop/products/cover-glasses-rectangles-promo-22/12544GP#coverglass |
| Fisherbrand Sterile Syringes for Single Use | Fisher Scientific | 14-955-458 | https://www.fishersci.com/shop/products/sterile-syringes-single-use-12/14955458 |
| Gibco RPMI 1640 Medium | Gibco | 11875093 | https://www.thermofisher.com/order/catalog/product/11875093 |
| Image-iT Fixative Solution (4% formaldehyde, methanol-free) | Invitrogen | FB002 | https://www.thermofisher.com/order/catalog/product/FB002 |
| ImageJ Fiji | ImageJ | ImageJ Fiji | https://imagej.net/downloads |
| Immersion Oil F 30 cc | Nikon | MXA22168 | https://www.microscope.healthcare.nikon. com/products/accessories/immersion-oil/specifications |
| Large-Capacity Reach-In CO2 Incubator, 821 L, Polished Stainless Steel | Thermo Scientific | 3950 | https://www.thermofisher.com/order/catalog/product/3950 |
| Laxco LMC-3000 Series Brightfield Compound Microscope System | Laxco | LMC3BF1 | https://www.fishersci.com/shop/products/lmc-3000-series-brightfield-compound-microscope-system-8/LMC3BF1 |
| Masterflex Fitting, Nylon, Straight, Male Luer Lock to Hose Barb Adapters, 1/16" ID; 25/PK | Masterflex | ZY-45505-31 | https://www.masterflex.com/i/masterflex-fitting-nylon-straight-male-luer-lock-to-hose-barb-adapters-1-16-id-25-pk/4550531?PubID=ZY&persist=true&ip=no& gclid=Cj0KCQiA3rKQBhCNARIsAC UEW_Zb5yXy1em6bGs0a9KFOk5k pdlkHCvAEslHumdqcnlwSN0MdR0 udmwaAuDHEALw_wcB |
| Microsoft Excel | Microsoft | 0016 | https://www.microsoft.com/en-us/download/details.aspx?id=56547 |
| National Target All-Plastic Disposable Syringes | Thermo Scientific | 03-377-24 | https://www.fishersci.com/shop/products/national-target-all-plastic-disposable-syringes/0337724#tab8 |
| NCI-H441 Human Epithelial Lung Cells | American Type Culture Collection (ATCC) | HTB-174 | https://www.atcc.org/products/htb-174 |
| NE-1600 Six Channel Programmable Syringe Pump | New Era Pump Systems | NE-1600 | https://www.syringepump.com/NE-16001800.php |
| NIS Elements AR | Nikon | NIS Elements AR | https://www.microscope.healthcare.nikon. com/products/software/nis-elements/nis-elements-advanced-research |
| NucBlue Live ReadyProbes Reagent (Hoechst 33342) | Invitrogen | R37605 | https://www.thermofisher.com/order/catalog/product/R37605?SID=srch-srp-R37605 |
| Nunc Lab-Tek Chambered Coverglass | Thermo Scientific | 155411 | https://www.thermofisher.com/order/catalog/product/155361 |
| Parafilm M Wrapping Film | Fisher Scientific | S37441 | https://www.fishersci.com/shop/products/parafilm-m-wrapping-film-3/S37441 |
| PendoTech 3-Way Stopcock, Polysulfone, Male/Female Luer Inlet x Female Luer Branch | PendoTech | ZY-19406-49 | https://www.masterflex.com/i/pendotech-3-way-stopcock-polysulfone-male-female-luer-inlet-x-female-luer-branch/1940649 |
| Phosphate Buffered Solution (PBS), pH 7.4 | Gibco | 10010023 | https://www.thermofisher.com/order/catalog/product/10010023 |
| Poly-D-Lysine | Gibco | A3890401 | https://www.thermofisher.com/order/catalog/product/A3890401#/A3890401 |
| Reynolds Aluminum Wrap Foil | Reynolds | 458742928317 | https://www.amazon.com/Reynolds-Wrap-Aluminum-Foil-Square/dp/B00UNT0Y2M |
| Saponin | Millipore Sigma (Sigma Aldrich) | 47036 | https://www.sigmaaldrich.com/US/en/product/sigma/47036 |
| SlowFade Glass Soft-set Antifade Mountant | Invitrogen | S36917-5X2ML | https://www.thermofisher.com/order/catalog/product/S36917-5X2ML |
| Thermo Scientific 1300 Series Class II, Type A2 Biological Safety Cabinet Package | Thermo Scientific | 13-100-752PM | https://www.fishersci.com/shop/products/1300-series-class-ii-type-a2-biological-safety-cabinet-package-promo/p-9049003#?keyword=biosafety%20hood |
| Tygon Transfer Tubing, BioPharm Platinum-Cured Silicone, 1/16" ID x 1/8" OD; 50 Ft | Cole-Parmer | EW-95702-01 | https://www.coleparmer.com/i/tygon-transfer-tubing-biopharm-platinum-cured-silicone-1-16-id-x-1-8-od-50-ft/9570201?searchterm=95702-01 |
Odniesienia
- Matthay, M. A., et al. Acute respiratory distress syndrome. Nature Reviews Disease Primers. 5, 18 (2019).
- Rawal, G., Yadav, S., Kumar, R. Acute respiratory distress syndrome: An update and Review. Journal of Translational Internal Medicine. 6 (2), 74-77 (2018).
- Bilek, A. M., Dee, K. C., Gaver, D. P. Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. Journal of Applied Physiology. 94 (2), 770-783 (2003).
- Modrykamien, A. M., Gupta, P. The acute respiratory distress syndrome. Baylor University Medical Center Proceedings. 28 (2), 163-171 (2017).
- Jacob, A. -. M., Gaver, D. P. Atelectrauma disrupts pulmonary epithelial barrier integrity and alters the distribution of tight junction proteins ZO-1 and Claudin 4. Journal of Applied Physiology. 113 (9), 1377-1387 (2012).
- Kay, S. S., Bilek, A. M., Dee, K. C., Gaver, D. P. Pressure gradient, not exposure duration, determines the extent of epithelial cell damage in a model of pulmonary airway reopening. Journal of Applied Physiology. 97 (1), 269-276 (2004).
- Jacob, A. M., Gaver, D. P. An investigation of the influence of cell topography on epithelial mechanical stresses during pulmonary airway reopening. Physics of Fluids. 17 (3), 031502 (1994).
- Gaver, D. P., et al. The POOR get POORer: A hypothesis for the pathogenesis of ventilator-induced lung injury. American Journal of Respiratory and Critical Care Medicine. 202 (8), 1081-1087 (2020).
- Jain, P., et al. Reconstruction of ultra-thin alveolar-capillary basement membrane mimics. Advanced Biology. 5 (8), 2000427 (2021).
- Byrne, M. B., Leslie, M. T., Gaskins, H. R., Kenis, P. J. A. Methods to study the tumor microenvironment under controlled oxygen conditions. Trends in Biotechnology. 32 (11), 556-563 (2014).
- Szulcek, R., Bogaard, H. J., van Nieuw Amerongen, G. P. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. Journal of Visualized Experiments. (85), e51300 (2014).
- Jaccard, N., et al. Automated method for the rapid and precise estimation of adherent cell culture characteristics from phase contrast microscopy images. Biotechnology and Bioengineering. 111 (3), 504-517 (2013).
- Hagiyama, M., et al. Modest static pressure suppresses columnar epithelial cell growth in association with cell shape and cytoskeletal modifications. Frontiers in Physiology. 8, 00997 (2017).
- Srinivasan, M., Sedmak, D., Jewell, S. Effect of fixatives and tissue processing on the content and integrity of Nucleic Acids. The American Journal of Pathology. 161 (6), 1961-1971 (2002).
- Zhu, L., Rajendram, M., Huang, K. C. Effects of fixation on bacterial cellular dimensions and integrity. Iscience. 24 (4), 102348 (2021).
- Lust, R. M. . The Pulmonary System. XPharm: The Comprehensive Pharmacology Reference. , 1-6 (2007).
- EpilogueLaser. FusionSeries: Pro & Edge Laser System Manual and Original Instructions. EpilogueLaser. , (2022).
- Chitnis, D. S., Katara, G., Hemvani, N., Chitnis, S., Chitnis, V. Surface disinfection by exposure to germicidal UV light. Indian Journal of Medical Microbiology. 26 (3), 241 (2008).
- Sandell, L., Sakai, D. Mammalian cell culture. Current Protocols Essential Laboratory Techniques. 5 (1), 4 (2011).
- New Era Pump Systems. Multi-Phaser Programmable Syringe Pump: NE-1000 Series User Manual. New Era Pump Systems. , (2014).
- Thermo Fisher Scientific. Safety Data Sheet: Image-iT Fixative Solution (4% formaldehyde, methanol-free). Thermo Fisher Scientific. , (2018).
- Thavarajah, R., Mudimbaimannar, V. K., Rao, U. K., Ranganathan, K., Elizabeth, J. Chemical and physical basics of routine formaldehyde fixation. Journal of Oral and Maxillofacial Pathology. 16 (3), 400-405 (2012).
- Jamur, M. C., Oliver, C. Permeabilization of cell membranes. Immunocytochemical Methods and Protocols. 588, 63-66 (2009).
- Thermo Fisher Scientific. ActinGreen 488 ReadyProbes Reagent Protocol. Thermo Fisher Scientific. , (2022).
- Slowfade Glass soft-set Antifade Mountant. Thermo Fisher Scientific Available from: https://www.thermofisher.com/order/catalog/product/S36917-5X2ML?SID=srch-hj-S36917-5X2ML (2022)
- Ravikumar, S., Surekha, R., Thavarajah, R. Mounting media: An overview. Journal of Dr. NTR University of Health Sciences. 3 (5), 1-8 (2014).
- Shihan, M. H., Novo, S. G., Le Marchand, S. J., Wang, Y., Duncan, M. K. A simple method for quantitating confocal fluorescent images. Biochemistry and Biophysics Reports. 25, 100916 (2021).
- North, A. J. Seeing is believing? A beginners' guide to practical pitfalls in image acquisition. Journal of Cell Biology. 172 (1), 9-18 (2006).
- Ferriera, F., Rasband, W. ImageJ User Guide. National Institutes of Health. , (2012).
- Halldorsson, S., Lucumi, E., Gómez-Sjöberg, R., Fleming, R. M. T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosensors and Bioelectronics. 63, 218-231 (2015).
- Smith, H. S., Riggs, J. L., Mosesson, M. W. Production of fibronectin by human epithelial cells in culture. American Association for Cancer Research. 39 (10), 4138-4144 (1979).
- Sieck, G. C., Mantilla, C. B., Prakash, Y. S. Volume measurements in confocal microscopy. Methods in Enzymology. 307, 296-315 (1999).
- Heijink, I. H., et al. Characterisation of cell adhesion in airway epithelial cell types using electric cell-substrate impedance sensing. European Respiratory Journal. 35 (4), 894-903 (2009).
- Zhang, X., Wang, W., Li, F., Voiculescu, I. Stretchable impedance sensor for mammalian cell proliferation measurements. Lab on a Chip. 17 (12), 2054-2066 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone