Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Method for Whole Mount Antibody Staining in Chick
W tym Artykule
Podsumowanie
This video demonstrates whole mount immunohistochemistry, a method by which the spatial and temporal expression pattern of an antigen can be visualized in young chick embryos. This method was originally introduced by Jane Dodd and Tom Jessell.
Streszczenie
Protokół
I. Schematic Overview:
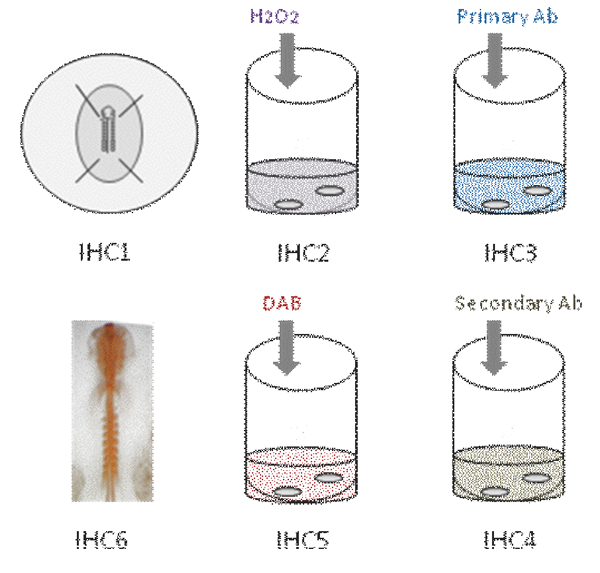
This video demonstrates the different steps in whole mount immunohistochemistry in chick embryo. First, the embryo is fixed in PFA [IHC1]. Then, endogenous peroxidase activity is quenched [IHC2]. The embryo is then incubated in primary antibody [IHC3]. After several washes, the embryo is incubated in secondary antibody [IHC4]; Color reaction is revealed using DAB [IHC5] and antibody staining appears orange [IHC6].
Part 1: Fixing the embryos
- To perform whole mount immunohistochemistry on chick embryos, first open an egg by tapping the shell with forceps and removing pieces of the shell.
- Remove the thick albumin with forceps, and tilt the yolk sac with coarse forceps so that the embryo faces upwards.
- Using fine scissors, cut a square of yolk sac around the embryo. Remove the embryo from the yolk with a spoon, and place in a dish containing PBS.
- Under a dissection microscope, carefully remove the membranes and yolk and the embryo transfer to a fresh dish containing PBS.
- Pin the embryo down with forceps and insect pins, and aspirate the PBS. Replace this with 4% PFA in PBS, and allow the embryo to fix 1h at RT.
Part 2: Preparing embryos for antibody step
- To minimize potential microbial contamination, use ddH2O in all following steps.
- After the embryo is fixed, aspirate the fixative solution and dispose properly as chemical waste. Fill the dish with PBS.
- next, using a microdissection knife, cut a square around embryo to remove extraembryonic membranes. Remove insect pins with forceps.
- After the pins have been removed, use a blunt end Pasteur pipette to transfer the embryo to a scintillation vial containing with PBT (PBS pH 7.4, 0.5% Triton X).
- Wash the embryo with PBT, 3 times for 10 minutes each.
- Remove PBT from the scintillation vial, and replace with PBTx containing 0.3% H2O2 in order to inactivate potential endogenous peroxidase.
- Incubate for 2 hr at RT on nutator.
- Wash embryo with PBT, 3 times for 10 minutes each, then 3 times for 30 minutes each.
Part 3: Antibody incubation
- To stain the embryo with antibody, start by washing the embryo for one hour in blocking buffer or 1% BSA/1% NGS/PBT (we use NGS because the secondary antibody is raised in goat). Incubate on nutator for one hour at RT.
- Dilute the primary antibody 1:1 in blocking buffer, and incubate embryo in this solution for 2 days at 4°C on nutator. Primary antibody dilution factor depends on the choice of primary antibody. Here, we use an antibody from the Developmental Studies Hybridoma Bank, which is provided as supernatent . We dilute it 1:1 in blocking buffer.
- next, perform 3 10-minute washes in PBT, followed by 3 1-hour washes in PBT.
- After washing, dilute the secondary antibody 1:2500 in blocking buffer (in this case peroxidase conjugated-goat anti-mouse IgG (H+L), and incubate embryo in this solution O/N at 4°C on nutator.
- Perform 3 10-minute washes in PBT, followed by 3 1-hour washes in PBT.
Part 3: Color reaction
- To develop the color reaction of the stained embryo, first perform 2 20-minute washes in Tris buffer (100mM Tris HCl, pH 7.4).
- Meanwhile dissolve DAB substrate (3,3’-diaminobenzidine tetrahydrochloride) in Tris buffer at 500μg/ml under fume hood; keep solution in dark, on ice.
- Remove last Tris buffer wash from the vial containing embryo and replace with 5ml DAB solution from step 3.2. Keep in dark on nutator for 20 mn. Dispose of Eppendorf tip in bucket containing a 10% bleach solution in order to decontaminate DAB.
- Meanwhile prepare a 0.3% stock H2O2 in dH2O on ice.
- Add 50μl stock H2O2 to vial containing embryos in DAB. Keep in dark. After 1-2 minutes, monitor reaction under microscope.
Part 4: Embryo processing for photography and histology
- When color reaction is complete, dispose of DAB in bleach bucket. Replace with 5 ml tap water.
- Perform 2 10-minute washes in PBS.
- To process for photography and wax sectioning, dehydrate in an ethanol series (25%, 50%, 75% and 100%) for 10 minutes each.
- Replace the ethanol with 0.5 - 1 ml cedar wood oil (this will make the embryo translucent). Process for photography in cedar wood oil.
- Following photography, return embryo to vial and replace the cedar wood oil with 100% ethanol. Repeat ethanol step.
- Replace ethanol with 5% Fast green FCF in 100% ethanol, and incubate for 3 minutes (this step will make embryos visible for histology). Replace Fast green FCF solution with 100% ethanol and process for histology.
Representative Results:
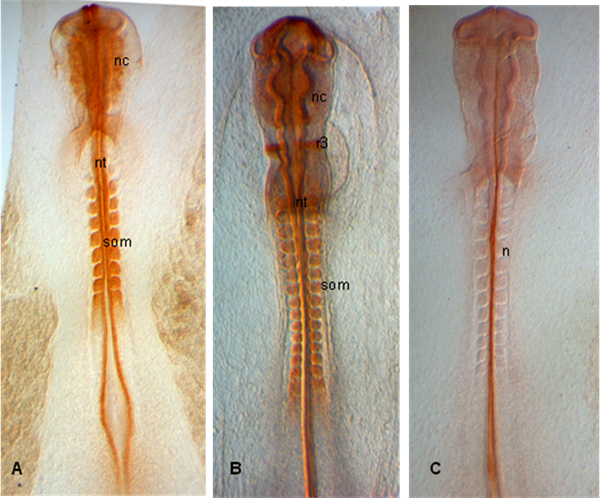
In the examples shown below, embryos are dissected at stages HH 10 (A), 12 (B) and 11 (C); Embryos show expression of PAX 7 in emerging neural crestas well as in somites and neural tube (A,B); In (C), notochord is labelled with 15.3B9 (Not-1) (Antibodies provided by Developmental Studies Hybridoma Bank).
Dyskusje
This video demonstrates the different steps in performing whole-mount antibody staining in young chick embryos. This protocol is essentially used for the spatial and temporal characterization of novel antibodies in chick 2,3, as well as for the use of known antigenic markers to determine embryonic malformations following insult 4.
Podziękowania
The monoclonal antibodies (15.3B9, PAX7) developed by ( J. Dodd, T.M Jessell and A. Kawakami) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological sciences, Iowa. D.P is recipient of Ruth Kirschstein Award 1F32 DA021977-01A1 from the National Institute on Drug Abuse. This work was supported by the Margaret M. Alkek Foundation to RHF.
Materiały
| Name | Company | Catalog Number | Comments | |
| Eggs | Charles River Laboratories | Premium Fertile | Fertilized, HH4 (16 hr) | |
| Stereomicroscope | Microscope | Leica Microsystems | MZ9.5 or similar | |
| Marsh Automatic Incubator | Other | Lyon | RX | |
| Curved Forceps (1) | Tool | Electron Microscopy Sciences | 72991-4C | |
| Forceps (2) | Tool | Fine Science Tools | 11002-13 | |
| Fine scissors | Tool | Fine Science Tools | 14161-10 | |
| Plastic dishes | Tool | Falcon BD | 353001 | |
| Rubber Bulb | Tool | Electron Microscopy Sciences | 70980 | |
| Pasteur Capillary Pipette | Tool | Electron Microscopy Sciences | 70950-12 | round edge under flame |
| Microdissecting knife | Tool | Fine Science Tools | 10056-12 | Use to cut embryo from surrounding membranes following fixation |
| Sylgard 184 Silicon Elastomer Curing Agent and Base | Reagent | Dow Corning | 0001986475 | Mix 1 part Curing Agent, 9 parts Base; set O/N at 37C |
| 16% PFA | Reagent | Electron Microscopy Sciences | 15710 | |
| 30% H2O2 | Reagent | Sigma-Aldrich | H1009 | |
| BSA | Reagent | Sigma-Aldrich | A3803 | |
| NGS | Reagent | Jackson ImmunoResearch | 005-000-121 | |
| Primary Antibodies | Reagent | Developmental Studies Hybridoma Bank | 4G11, 15.3B9, PAX7 | For these antibodies, investgators used mouse donnors |
| Peroxidase conjugated-Goat Anti-Mouse IgG (H+L) | Reagent | Jackson ImmunoResearch | 115-035-003 | |
| 3,3’-diaminobenzidine tetrahydrochloride | Reagent | Pierce, Thermo Scientific | 34001 | Store at -20; Allow bottle to warm to RT before use. |
| Cedar wood oil | Reagent | Sigma-Aldrich | W522503 | |
| Fast green FCF | Reagent | Sigma-Aldrich | F7252 | |
| Minuten pins 0.2mm diam | Fine Science Tools | 26002-20 |
Odniesienia
- Yamada, T., Placzek, M., Tanaka, H., Dodd, J., Jessell, T. M. Control of cell pattern in the developing nervous system: Polarizing activity of the floor plate and notochord. Cell. 64, 635-647 (1991).
- Streit, A., Stern, C. D., Thery, C., Ireland, G. W., Aparicio, S., Sharpe, M. J., Gherardi, E. A role for HGF/SF in neural induction and its expression in Hensen's node during gastrulation. Development. 121, 813-824 (1995).
- Basch, M., Bronner-Fraser, M., Garcia-Castro, M. Specification of neural crest occurs during gastrulation and requires Pax7. Nature. 441, 218-222 (2006).
- Psychoyos, D., Stern, C. D. Restoration of the organizer after radical ablation of Hensen's node and the anterior primitive streak in the chick embryo. Development. 122, 3263-3273 (1996).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone