Method Article
Single-Molecule Fluorescence Visualization of DNA Polymerase Dynamics at G-Quadruplexes
In This Article
Summary
This protocol outlines a fluorescence microscopy-based single-molecule DNA replication assay, enabling real-time visualization of interactions between DNA polymerases and obstacles such as G-quadruplex structures.
Abstract
The ability of proteins involved in eukaryotic DNA replication to overcome obstacles — such as protein and DNA 'roadblocks' — is critical for ensuring faithful genome duplication. G-quadruplexes are higher-order nucleic acid structures that form in guanine-rich regions of DNA and have been shown to act as obstacles, interfering with genomic maintenance pathways. This study introduces a real-time, fluorescence microscopy-based method to observe DNA polymerase interactions with G-quadruplex structures. Short, primed DNA oligonucleotides containing a G-quadruplex were immobilized on functionalized glass coverslips within a microfluidic flow cell. Fluorescently labeled DNA polymerases were introduced, allowing their behavior and stoichiometry to be monitored over time. This approach enabled the observation of polymerase behavior as it was stalled by a G-quadruplex. Specifically, using fluorescently labeled yeast polymerase δ, it was found that upon encountering a G-quadruplex, the polymerase undergoes a continuous cycle of binding and unbinding. This single-molecule assay can be adapted to study interactions between various DNA-maintenance proteins and obstacles on the DNA substrate.
Introduction
DNA polymerases are enzymes that catalyze the incorporation of nucleoside triphosphates to duplicate DNA1,2,3,4,5. As such, they play a key role in essential DNA maintenance processes, including DNA replication6,7,8 and repair9,10,11,12. DNA polymerases must replicate the genome accurately and efficiently to ensure genomic integrity, preventing the accumulation of mutations in the genome. During synthesis, polymerases often encounter "roadblocks" such as DNA-bound proteins or secondary DNA structures13. These roadblocks can slow or even block polymerase progression14. Overcoming these roadblocks is important to ensure faithful genome duplication, as failure to do so can lead to genomic instability15,16.
One major class of roadblocks is G-quadruplexes (G4), non-canonical secondary DNA structures that have been shown to form in guanine-rich sequences within the human genome17. There are over 700,000 different sequences in the human genome capable of forming a G4, including regions within telomeres and oncogene promoters18. These DNA structures adopt various conformations depending on the nucleotide sequence, length, and bound metal cation19,20,21. This diversity means polymerases must overcome a range of different G4 topologies, potentially with varying degrees of efficiency. Failure of polymerases to overcome or bypass a G4 structure has been shown to impede replication fork progression in vivo, leading to genomic instability22. In vitro studies have shown that G4 structures can stall or completely block yeast polymerases23,24,25,26,27. The ability of G4 structures to stall or block DNA polymerases is entirely dependent on their kinetic and thermodynamic stability, with some polymerases being able to unfold certain G4's28. While these studies provide insight into the polymerases' ability to overcome G-quadruplex roadblocks, they lack the ability to directly visualize the polymerase's behavior upon encountering a G4. The fate of the polymerases - whether they remain bound, fall off, or dynamically exchange - determines what downstream processes are accessible to resolve the G4.
In this study, a fluorescence-based single-molecule microscopy assay was developed to directly visualize and monitor DNA polymerase binding with G4 structures in real-time. This assay involves tethering G4-forming DNA templates to a biotinylated coverslip in a microfluidic flow cell, where fluorescently labeled DNA polymerases can be introduced to initiate DNA synthesis. By measuring the fluorescence of the polymerases over time, their behavior upon encountering a G4 structure can be directly observed. The G4 structure found in the c-MYC cancer oncogene was chosen for this assay due to its high level of stability. This protocol can now be adapted to catalog the behavior of a variety of polymerases across all domains of life associated with different G4 topologies and stabilities. This assay offers an innovative and high-throughput approach to elucidate the mechanisms by which DNA polymerases navigate DNA obstacles, providing a powerful tool for advancing the understanding of polymerase dynamics.
Protocol
Details of the reagents and the equipment used are listed in the Table of Materials.
1. Circular dichroism spectroscopy
NOTE: Prior to developing the assay, it was necessary to conduct circular dichroism (CD) spectroscopy on the selected G4 sequence to ensure correct folding. The 22 nt sequence (5′-TGAGGGTGGGGAGGGTGGGGAA-3′) forms the G4 structure derived from the c-MYC cancer oncogene. CD spectroscopy was also performed on a control sequence (5′- TGAGTGTGAAGACGATGTAGAA -3) which has key nucleotides altered that prevent G4 formation.
- To prepare the DNA templates for CD spectroscopy, add 40 µL of 100 µM of the 22 nt templates to 360 µL of Tris-HCl (pH 8.0), ethylenediaminetetraacetic acid (EDTA) (TE) buffer supplemented with 200 mM of potassium chloride (KCl). A 10 µM of final DNA concentration is desired for CD spectroscopy experiments.
NOTE: The presence of K+ ions is essential for stabilizing the G4 structure in the template. - Heat the DNA solutions to 95 °C in a digital dry bath for 15 min. Once complete, cool the solutions slowly overnight by placing the heating block into polystyrene. This slow cooling process is responsible for the folding of the G4.
- Transfer 400 µL of the G4-forming and control sequence solutions to a 0.1 cm pathlength quartz cuvette using a gas-tight syringe. Fill the cuvette slowly to avoid the formation of air bubbles.
- Measure the CD spectrum between 200 nm and 400 nm using a spectropolarimeter at 25 °C. The parameters and conditions for this measurement have been described previously29. Results can be found in Supplementary Figure 1.
NOTE: Ensure to record the CD spectrum for the buffer alone to act as a blank.
2. Preparation of DNA templates
NOTE: The templates capable of being replicated are short, 100 nt primed linear templates prepared using standard molecular biology techniques. The G4-forming template (5′-GAATTACATTTAAATTTTACACAGATACAGTCAATGAGAA
CTTCCAGGCGTAACGAGAGCACGGGGTGGGAGGGGT
GGGACCTTAGCTTCGAGTTCCGAT-3′) contains the sequence capable of forming a MYC-derived G-quadruplex (from step 1) in its center. The control template (5′-GAATTACATTTAAATTTTACACAGATACAGTCAATGAGA
ACTTCCAGGCGTAACGAGAGCACGATGTAGCAGAACT
GTGACCTTAGCTTCGAGTTCCGAT-3′) has the same control region (from step 1) also in its center.
- To anneal the primer to the G4-forming and control DNA templates, mix 20 µM of the primer (5′-Biotin-AF647-TCTTAATGTAAATTTAAAATGTGTC-3′) to 20 µM of the 100 nt templates in TE buffer supplemented with 200 mM KCl.
- Heat the DNA template solutions to 95 °C in a digital dry bath for 15 min. Once complete, cool the solutions slowly overnight by placing the heating block into polystyrene to allow folding of the G4 and annealing of the two strands.
3. Labeling of DNA polymerase with AF647
- Labeling the polymerase with the dye
NOTE: The yeast Pol δ was expressed and purified as previously described30. The polymerase then needed to be labeled with the Alexa Fluor NHS ester mono-reactive dye (AF647) to allow for visualization on the single-molecule level. This was completed as previously described5.- To label the N-terminus, incubate a 3-fold excess of the AF647 dye with 320 µL of 58.5 µM Pol δ in a buffer containing 30 mM of Tris-HCl (pH 7.6), 2 mM of dithiothreitol (DTT), 300 mM of sodium chloride (NaCl), 50 mM of potassium glutamate, and 10% (v/v) glycerol for 10 min at 4 °C. Gently rotate the solution to ensure thorough mixing and improve labeling efficiency.
NOTE: The protein sequence may contain additional primary amines in lysine amino acids. By adjusting the reaction conditions, such as the pH, the label can be preferentially attached to the N-terminus as opposed to the lysine residues. This may require further optimization to increase the labeling efficiency. - To prepare the 0.5 mL spin desalting columns (7 K molecular weight cut off), first centrifuge the columns at 1500 x g for 1 min to remove the storage solution. Discard the solutions deposited in the collection tube after centrifugation.
NOTE: Ensure to loosen the cap of the spin columns during the centrifugation steps. This will ensure the columns are properly eluted. - Equilibrate the columns in a buffer containing 30 mM of Tris-HCl (pH 7.6), 300 mM of NaCl, 3 mM of DTT, 50 mM of potassium glutamate, and 5% (v/v) glycerol by adding 300 µL of buffer to the top of the column. Centrifuge the column at 1500 x g for 1 min and discard the flow-through. Repeat this process two additional times.
- Apply 200 µL of each Pol δ sample to the column. Centrifuge the columns at 1500 x g for 2 min and collect the purified Pol δ in a 1.5 mL microcentrifuge tube.
- Freeze the purified fractions in liquid N2 and store the aliquots at -80 °C to prevent protein degradation over time.
- To label the N-terminus, incubate a 3-fold excess of the AF647 dye with 320 µL of 58.5 µM Pol δ in a buffer containing 30 mM of Tris-HCl (pH 7.6), 2 mM of dithiothreitol (DTT), 300 mM of sodium chloride (NaCl), 50 mM of potassium glutamate, and 10% (v/v) glycerol for 10 min at 4 °C. Gently rotate the solution to ensure thorough mixing and improve labeling efficiency.
- Determining labeling efficiency
- Load 2 µL of the now AF647-labeled Pol δ sample onto a UV-vis spectrophotometer31.
NOTE: Ensure a blank of the Pol δ buffer is measured first. - Measure the excitation wavelengths of 650 nm for the AF647 dye and 280 nm for the Pol δ using the spectrophotometer.
- Using the Beer-Lambert law and the molar extinction coefficients of 270,000 cm-1M-1 for the AF647 dye and 195,960 cm-1M-1 for the polymerase, calculate the labeling efficiency.
NOTE: The labeling efficiency determined for the AF647-labeled yeast Pol δ used in this assay was calculated to be approximately 67%.
- Load 2 µL of the now AF647-labeled Pol δ sample onto a UV-vis spectrophotometer31.
4. Ensemble primer extension assay
NOTE: Before performing single-molecule replication experiments, it is necessary to confirm the DNA polymerase is blocked by the G-quadruplex via bulk replication assays.
- Set the temperature of a heating block within a digital dry bath to 30 °C. This will ensure the maximal activity of the yeast Pol δ.
- Prepare "master mixes" for the G4-forming and control templates in replication buffer (25 mM of Tris-HCl (pH 7.6), 10 mM of magnesium acetate, 50 mM of potassium glutamate, 40 µg/mL bovine serum albumin (BSA), 0.1 mM of EDTA, 5 mM of DTT, and 0.0025% (v/v) Tween-20). Supplement each mix with 1 mM of DTT, 250 µM each of dTTP, dCTP, dATP, dGTP (dNTPs), and 10 nM of the respective DNA template (G4-forming or control). Place these in a heating block to ensure they reach 30 °C.
- Quench 12 µL of each master mix with 12 µL of formamide loading buffer (80% (w/v) formamide, 10 mM of EDTA) to act as the T = 0 min control.
- To initiate DNA synthesis, add AF647-labeled yeast Pol δ to each master mix (20 nM final concentration). Thoroughly mix to ensure the DNA polymerases replicate the templates with maximum efficiency.
NOTE: Keep the yeast Pol δ on ice during use to preserve enzyme activity. - After 30 s, 60 s, and 180 s, remove 12 µL of each master mix and quench with 12 µL of formamide loading buffer. The 1:1 ratio of the master mix to the formamide loading buffer ensures the replication reaction cannot continue once quenched.
- Remove the now quenched solutions from the 30 °C heating block and place them into a 98 °C heating block instead for denaturing the dsDNA to ssDNA. Once 10 min have elapsed, bring the entire heating block to the gel tank for loading of the solutions into a denaturing 15% tris-borate-EDTA (TBE)-urea polyacrylamide (PAGE) gel32.
NOTE: The PAGE gel needs to be warmed prior to the loading of the samples by running the gel electrophoresis at 180 V for 30 min in 1x TBE buffer. Once complete, rinse each well to remove urea. - Load 15 µL of each sample onto the gel alongside a ladder containing 0.02 µM of 20 nt, 60 nt, and 100 nt labeled oligonucleotides in formamide loading buffer.
- Run the gel for 60 min at 180 V in 1x TBE buffer to ensure complete separation.
- Image the gel in the Cy5 channel of a biomolecular imager. This will allow the replicated DNA strand containing the AF-647 label to be measured.
5. Single-molecule fluorescence microscopy
- Coverslip functionalization
NOTE: To attach the DNA templates to the glass coverslip, it must first be functionalized with aminosilane, followed by the tethering of biotinylated PEG molecules. This process minimizes the nonspecific interactions between the DNA and/or proteins and the surface.- Clean the coverslips (24 x 24 mm) by inserting them into staining jars and pouring in ethanol. Sonicate the jars for 30 min before rinsing the coverslips with pure water. Repeat this process with 1 M potassium hydroxide (KOH) before rinsing again. Repeat these cleaning steps once more.
- Rinse and fill a new jar with acetone and place the coverslips inside. Disseminate 3-aminopropyltriethoxysilane into the jar to form a 2% v/v solution. Agitate the jar for 3 min before quenching the reaction with a large excess of water.
- Dry the coverslips with N2 and place them individually onto a water-filled humid box. This will prevent the coverslips from drying out.
- Prepare a 1:25 Biotin-PEG-SVA:mPEG-SVA ester in 100 mM of NaHCO3 (pH 8.2). Vortex this mixture for 20 s to ensure thorough mixing.
- Pipette 40 µL of the PEG solution onto a dry coverslip. Place another coverslip on top to "sandwich" the solution between the two coverslips. This will functionalize the faces of the coverslip on the inside. Repeat for all coverslips.
- Incubate the coverslips in the dark for 3 h. After incubation, separate the coverslip pairs, rinse them with excess water, and dry them with compressed N2 gas.
- Repeat steps 4.1.4-4.1.5 to apply a second layer of PEG to the coverslips. Ensure to sandwich the previously functionalized sides together, so be careful not to flip them accidentally during the washing steps. Incubate the solutions in the dark overnight before rinsing and drying them.
- Store the coverslips in a vacuum to preserve their functionality. Functionalized coverslips are stable for a month when stored correctly.
- Preparation of microfluidic flow cells
NOTE: A microfluidic flow cell33 is constructed for single-molecule experiments (see Supplementary Figure 2). This will allow buffers, DNA templates, and proteins to come into contact with a functionalized coverslip (see step 5.1).- Remove a functionalized coverslip from a vacuum and place it onto a microtube rack partially filled with water (humid box). Mix 100 µL of a blocking buffer (50 mM of Tris-HCl (pH 7.6), 50 mM of KCl, 2% (v/v) Tween-20) with 25 µL of 1 mg/mL NeutrAvidin solution (10% PBS) and spread this onto the surface of the coverslip. Let this incubate for 15 min at room temperature in the humid box.
- Wash the coverslip in water and dry in N2. Remember that only one side of the coverslip is functionalized, so remember which side it is.
- Place custom-made polydimethylsiloxane (PDMS) block34 onto the coverslip within the flow cell holder. This will generate a 100 µm high and 1 mm wide flow channel. Polyethylene tubes (PE-60: 0.76 mm inlet diameter and 1.22 mm outer diameter) can then be inserted into the holes in the flow cell to provide access to buffers and substrates.
- Single-molecule fluorescence imaging
NOTE: These experiments are to be performed with both the G4-forming and control templates. This will allow both conditions (blocking of the Pol δ by a G4 structure and no blocking) to be visualized on the single-molecule level using total internal reflection (TIRF) microscopy.- Heat 1 mL aliquots of a Tween blocking buffer (50 mM of Tris-HCl (pH 7.6), 50 mM of KCl, 2% (v/v) Tween-20) and 500 µL aliquots of a wash buffer (25 mM of Tris-HCl (pH 7.6), 10 mM of magnesium acetate, 250 mM of potassium glutamate, 40 µg/mL BSA, 0.1 mM of EDTA, 5 mM of DTT, 0.0025% (v/v) Tween-20) and replication buffer to 40 °C for 15 min. This will liberate the gases from the solutions. De-gas the solutions in a vacuum chamber for a further 15 min at 800 mbar below atmospheric pressure.
- Take the constructed flow cell (see step 4.2) and place it onto the stage of the microscope. After placing a drop of oil on the objective, raise the objective to meet the coverslip.
NOTE: Ensure the objective and flow cell have time to reach 31 °C. This will increase the activity of the yeast Pol δ and help ensure reproducible data. - Insert the inlet tube into the degassed Tween blocking buffer and connect the outlet to the syringe pump. Pull back on the syringe to draw the Tween blocking buffer through the tubing into the channel. Allow this buffer to incubate for at least 30 min to minimize nonspecific interactions.
- Flow in 200 µL degassed wash buffer into the channel at a rate of 100 µL/min. This will flush out the Tween blocking buffer.
- Dilute the DNA template solutions to 0.5 pM in 500 µL replication buffer. Flow 150 µL into the channel at a rate of 10 µL/min. Illuminate the sample using a 647nm laser at approximately 900 mWcm-2 at the sample plane to visualize individual DNA templates.
NOTE: If the field of view (FOV) with the sample is not completely in focus, the cover slip may not be sitting flush against the bottom of the constructed flow cell. Construct a new flow cell using a fresh coverslip if this occurs. - Once a sufficient density of spots (ca 1 spot per 10 µm2) is visible, flow in a fresh solution of replication buffer (supplemented with 1 mM of DTT) into the channel to wash out excess DNA. A volume of 250 µL is sufficient to completely remove all unbound DNA.
- Move to a new FOV and capture an image of the DNA to determine the degree of colocalization between the labeled polymerase and the DNA substrate. Once complete, increase the laser power to photobleach the remaining spots.
- Prepare a polymerase solution containing 1 mM of DTT, 250 µM of dNTPs, and 20 nM of AF647-labeled Pol δ in 200 µL of replication buffer. Flow in 100 µL of the polymerase solution at a rate of 5 µL/min into the channel.
- Once the sample is in focus and the TIRF angle has been adjusted, set the laser power of a 647 nm laser to approximately 900 mWcm-2 at the sample plane. Then, begin imaging the FOV for the desired length of time. Acquire between 1-5 frame/s for 10-20 min to capture all replication events.
- Data analysis
NOTE: All analyses were carried out using Python (v. 3.11.7). The custom code used for the data analysis is available here: https://github.com/Single-molecule-Biophysics-UOW/G-quadruplex_binding_analysis.- First, colocalize the DNA template spots with the fluorescent spots of the labeled yeast Pol δ. Two peaks were determined to be colocalized if their distance after drift correction was ≤ 3 pixels. Only the Pol δ spots that colocalize DNA templates are analyzed for their binding kinetics and overall behavioral trends.
- To determine binding event data, measure the intensity over time of each spot as they exhibit "on/off" behavior throughout the movie. This enables the determination of how many times the Pol δ binding occurs on a single DNA template.
NOTE: This analysis is done for both the G4-forming and control template experiments to compare the binding dynamics.
NOTE: Control experiments have been carried out, which ensure nonspecific binding events to the G-quadruplex or the template itself (23.2 s ± 4.9 s) are not included in the analysis of the binding events.
Results
For this assay, two DNA substrates with a fluorescently labeled 20-nt primer were designed: one containing a G4-forming sequence (Figure 1A, left) and one lacking this sequence (Figure 1A, right). To confirm that the G-quadruplex is an effective roadblock to polymerase activity, DNA synthesis by Pol δ was monitored on a denaturing PAGE gel. The activity of the purified labeled Pol δ on the DNA substrates was examined by gel electrophoresis. Figure 1B (left) shows that the fluorescently labeled Pol δ is unable to synthesize past the G-quadruplex. Before the initiation of synthesis (t = 0 min), a band corresponding to 20 nt is present, representing the labeled primer that has been denatured from the template strand. After 3 min, this 20-nt band was converted to a 60-nt band, indicating synthesis occurred on all DNA and confirming that the polymerase was completely blocked by the G-quadruplex structure. This blocking implies the polymerase could neither unfold nor bypass the structure. In contrast, the synthesis of a control template that does not contain the G-quadruplex-forming sequence (Figure 1A, right) produced a 100-nt band after 3 min (Figure 1B, right).
After confirming synthesis on the templates and efficient blocking by the G-quadruplex, these measurements were repeated on a single-molecule fluorescence microscope to monitor polymerase behavior. The DNA templates were tethered to functionalized coverslips (Figure 2A) in a microfluidic flow cell, and the position of each substrate was determined by visualizing the fluorescently labeled primer. Then, labeled Pol δ was loaded in the presence of dNTPs to initiate synthesis. Within a typical FOV, individual spots are tracked to quantify how often Pol δ binds and dissociates from the DNA (Figure 2B,C). By measuring the intensity as a function of time at each DNA substrate, single-molecule trajectories can be generated (Figure 2C, Supplementary Figure 3). The characteristic "dwell time", or the average duration in which Pol δ remains bound to the template, can be measured. For the G4 substrate, the dwell time was determined to be 6 s ± 2 s, while for the control substrate, it was 10 s ± 3 s (Figure 2D, Supplementary Figure 4). Additionally, for each trajectory, the number of times Pol δ binds to the template can be quantified. The number of binding events to the G4 substrate is much higher compared to the control template (Figure 2E). While there are instances of more than one binding event in the control template due to scholastic binding and unbinding, there is a clear increase in the number of binding events on average for the G4-forming template. This suggests that, after synthesis is halted by the G-quadruplex, the bound polymerase dissociates from the DNA before new polymerases from the solution engage a continuous cycle of binding and unbinding. Hence, this single-molecule assay provides unparalleled insight into how DNA polymerases respond to DNA roadblocks.
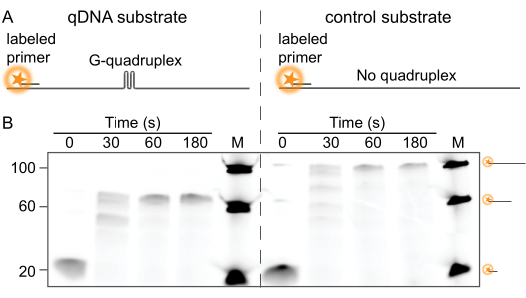
Figure 1: Ensemble primer extension assay. (A) Schematic representation of the DNA substrates. The G4 substrate (left) contains a G-quadruplex-forming sequence, whereas the control substrate (right) does not. (B) Ensemble DNA primase-extension assay of the primed G4 and control DNA substrates showing that fluorescently-labeled yeast Pol δ is fully blocked by the G-quadruplex. The PAGE gel shows the replication of the DNA templates over time, resulting in a shift of the labeled 20 nt primer into the 60-nt product for the G4 substrate (left), and the 100-nt product for the control template (right). M represents a ladder containing labeled 20 nt, 60 nt, and 100 nt oligos. Please click here to view a larger version of this figure.
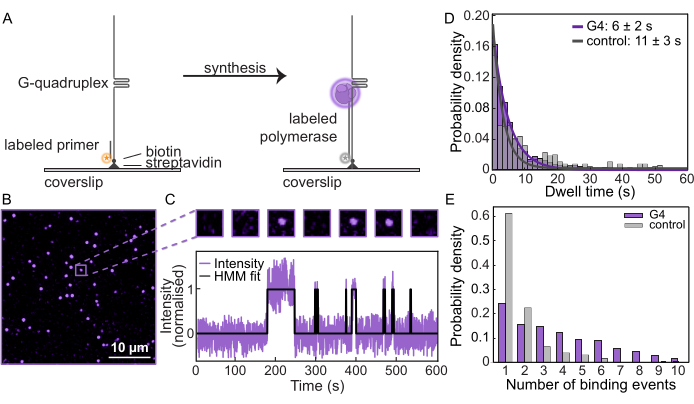
Figure 2: Single-molecule fluorescence microscopy. (A) Schematic representation of the single-molecule primer extension assay using AF647-labeled yeast Pol δ. (B) Typical FOV obtained from a single-molecule assay. Each spot represents a fluorescently labeled Pol δ binding to a DNA template. Scale bar: 10 µm. (C) (Top) Example molecule from the single-molecule assay showing on/off binding as the Pol δ exchanges. (Bottom) Single-molecule trajectory of the same molecule showing the intensity over the time course of the experiment. The black line represents the Hidden Markov Model (HMM) fit to the data. (D) Dwell time of Pol δ on the G4 substrate (purple) and the control substrate (gray). The lines represent exponential fits, giving a lifetime of 6 s ± 2 s on the G4 substrate and 10 s ± 3 s on the control substrate. (E) Number of Pol δ binding events to single DNA substrates. The median number of binding events on the G4 substrate (purple) is 3.5, compared to the control substrate 1 (gray). Please click here to view a larger version of this figure.
Supplementary Figure 1:CD spectroscopy. CD spectra of the G4-forming (purple) and control sequence (gray) in TE buffer containing 200 mM of KCl at 25 °C. The characteristic peak at 260 nm, followed by the negative peak at 240 nm, is characteristic of a parallel, intramolecular GQ. Please click here to download this File.
Supplementary Figure 2: Flow cell construction. (A) Schematic of the individual pieces of the flow cell. The square PDMS block sits on top of the microscope coverslip. These pieces are then fit together inside the lid and bottom of the flow cell holder. (B) The complete flow cell. Tubes can be inserted into each side of the PDMS block to form the channels through which buffers can be flowed through the device via microfluidics. Please click here to download this File.
Supplementary Figure 3: Single-molecule trajectories. (A) Example trajectory of a molecule undergoing a single binding event during the 10 min time frame of the experiment. (B) Example trajectory of a molecule undergoing zero binding events during the 10 min time frame of the experiment. The black line in (A) and (B) represents the Hidden Markov Model (HMM) fit to the data. Please click here to download this File.
Supplementary Figure 4: Dwell times. (A) Dwell time of Pol δ on the G4 substrate. The line represents the exponential fit, giving a lifetime of 6 s ± 2 s. (B) Dwell time of Pol δ on the control substrate. The exponential fit gives a lifetime of 10 s ± 3 s. Please click here to download this File.
Supplementary Figure 5: Photobleaching control. The plot shows the average time for an individual AF647-labeled yeast Pol δ enzyme to be photobleached by the 647 nm laser. The line represents the exponential fit, giving a lifetime of 39 ± 6 s. Please click here to download this File.
Discussion
Here, a single-molecule fluorescence-based assay has been described that provides insight into the behavior of a DNA polymerase as it encounters a G-quadruplex. While the protocols of DNA template generation, Pol δ labeling, and bulk DNA replication assays are all straightforward, executing single-molecule microscopy assays is more technically challenging. Due to the nature of single-molecule techniques, great care must be taken to avoid the introduction of dust, contamination, or air bubbles, as these will obscure the FOV and hamper data collection.
A limitation of single-molecule TIRF microscopy experiments is the photobleaching of the fluorophores that are covalently coupled to the biomolecules of interest. Photobleaching is an irreversible process leading to permanent fluorescence loss35. To mitigate this during experiments, it is essential to limit laser exposure duration, adjust laser intensity, and optimize imaging timing. These strategies help preserve fluorescence signals, ensuring more reliable and extended observation periods. By fine-tuning these parameters, the Pol δ signal remains for the duration of the measurement. To optimize laser power for the single-molecule fluorescence microscopy synthesis assays, it is advisable to measure the photobleaching rate by imaging the labeled polymerase on a clean glass coverslip. By systematically varying the laser power and assessing the photobleaching rate across the field of view, one can identify the optimal laser intensity that balances fluorophore signal strength with resistance to photobleaching (see Supplementary Figure 5).
The key advantage of this single-molecule approach over traditional ensemble-based methods is its ability to directly visualize when an individual DNA polymerase encounters and interacts with a G4 structure. Traditional ensemble-based methods (such as gel electrophoresis) have demonstrated the capacity for G4 structures to block DNA polymerases23,36,37. These techniques, however, fail to provide the real-time kinetic and mechanistic information of this interaction, which is necessary to disentangle different molecular kinetic steps and outcomes. Single-molecule techniques offer unparalleled insight into the kinetics, mechanisms, and behaviors of biomolecules often hidden by ensemble averaging38. It is now possible to see how DNA polymerases are acting in real-time - whether they exchange, stall, dissociate, or bypass DNA roadblocks39. With this protocol established, the identity of the G4 can easily be changed from the chosen parallel c-MYC structure to any parallel, anti-parallel, or hybrid topology. Applying this single-molecule assay will reveal whether the same DNA polymerases behave differently when encountering alternate G4 topologies. As such, single-molecule methods are vital for answering specific questions regarding how the body's proteins and DNA interact.
Through direct visualization of DNA polymerase interactions with G-quadruplexes, a previously uncharacterized exchange pathway for yeast Pol δ has been identified. This discovery suggests that the polymerase disengages upon encountering a G-quadruplex, awaiting the intervention of another protein to resolve the structure before reinitiating DNA synthesis. This protocol can be adapted to investigate interactions between various genome maintenance proteins and DNA obstacles, offering unparalleled insights into how cellular enzymes navigate genomic impediments. For example, this assay's roadblock can be altered from a G4 structure to a protein-DNA crosslink, a type of DNA lesion in which a protein is irreversibly covalently bound to DNA, acting as an obstacle for DNA replication40. Such examinations are crucial for understanding the fundamental processes of DNA replication, repair, and recombination. By enabling the study of DNA-protein dynamics at the molecular level, this assay provides a powerful tool for elucidating the mechanisms underlying genomic integrity.
Disclosures
The authors declare they have no competing financial interests.
Acknowledgements
N.K.-A. acknowledges the funding given by the Australian Government Research Training Program Scholarship. L.M.S. is grateful for the funding she has received from the National Health and Medical Research Council (Investigator Grant 2007778). J.S.L is grateful to be the recipient of a Discovery Early Career Award (DE240100780) and NHMRC Investigator EL1 (2025412) funded by the Australian Government. S.H.M is grateful to be the recipient of the Bruce Warren Molecular Horizons Ealy career fellowship.
Materials
| Name | Company | Catalog Number | Comments |
| 100 nt control DNA template | Integrated DNA Technologies | Primary control template in primer extension assays | |
| 100 nt G4-forming DNA template | Integrated DNA Technologies | Primary G4-forming template in primer extension assays | |
| 15% Mini-PROTEAN TBE-Urea Gel, 12 well, 20 µL | Bio-Rad | 4566055 | Gel electrophoresis |
| 20 nt labelled/biotinylated primer oligonucleotide | Integrated DNA Technologies | Required for primer extension of the 100 nt templates | |
| 22 nt control DNA template | Integrated DNA Technologies | Primary control template in circular dichroism spectroscopy experiments | |
| 22 nt G4-forming DNA template | Integrated DNA Technologies | Primary G4-forming template in circular dichroism spectroscopy experiments | |
| 24 x 24 mm coverslip | Marienfeld Superior | 100062 | Required for TIRF microscopy |
| 3-aminopropyltriethoxysilane | Thermo Fisher Scientific | A10668.22 | Coverslip functionalization |
| 647 nm laser | Coherent | 1196627 | Select wavelength to correspond to stain/dye of choice |
| 670 nm emission filter | Chroma Technology Corp | ET655lp | Ensures only the relevant wavelengths are let through to the detector |
| Amersham Typhoon 5 biomolecular imager | Cytiva | 29187191 | Gel analysis |
| Biotin-PEG-SVA (MW 5000) | Laysan Bio | BIO-PEG-SVA-5K-100MG | Coverslip functionalization |
| Deoxynucleotides triphosphate solution mix | Jena Bioscience | NU-1005L | Replication building blocks |
| Digital dry bath (115V) | Bio-Rad | 1660571 | Heating solutions |
| Dithiothreitol | Sigma-Aldrich | 646563 | Disulfide bond reduction |
| EM-CCD camera | Andor | iXon 897 | Capturing single-molecule movies |
| Formamide | Sigma-Aldrich | F9037 | DNA denaturing during ensemble DNA replication assays |
| Gas tight syringe, 1 mL | SGE | 21964 | Filling cuvette for circular dichroism spectroscopy |
| Inverted microscope with CFI Apo TIRF 100x oil-immersion objective | Nikon | Eclipse Ti-E | Facilitates single-molecule TIRF microscopy |
| J-810 Spectrophotometer | Jasco | J-810 | Measuring circular dichroism spectroscopy |
| mPEG-SVA (MW 5000) | Laysan Bio | MPEG-SVA-5K-100MG | Coverslip functionalization |
| NanoDrop One Spectrophotometer | Thermofisher Scientific | ND-ONE | Measuring protein and label concentrations |
| NeutrAvidin | Thermo Fisher Scientific | 31000 | Tethering DNA templates to the functionalized coverslips during single-molecule experiments |
| Polyethylene tubing | Instech | BTPE-60 | Needed for flow cell construction |
| Quartz cuvette, 10 mm | Starna Scientific | 1/Q/10/CD | Holds sample for circular dichroism spectroscopy |
| Syringe pump | Adelab Scientific | NE-1002X | Used to flow buffers and substrates through a microfluidic flow cell |
| Tris-EDTA buffer | Thermofisher Scientific | 93283 | DNA solution buffer |
| Yeast polymerase δ | Michael O'Donnell Lab | Replicating the DNA templates | |
| Zeba spin desalting column, 7 K MWCO, 0.5 mL | Thermo Fisher Scientific | 89882 | Polymerase purification |
References
- Sutton, M. D. Walker, G. C. Managing DNA polymerases: Coordinating DNA replication, DNA repair, and DNA recombination. Proc Natl Acad Sci U. S. A. 98 (15), 8342-8349 (2001).
- Garg, P. Burgers, P. M. J. DNA polymerases that propagate the eukaryotic DNA replication fork. Crit Rev Biochem Mol Biol. 40 (2), 115-128 (2005).
- Burgers, P. M. J. Eukaryotic DNA polymerases in DNA replication and DNA repair. Chromosoma. 107 (4), 218-227 (1998).
- Tsai, M.-D. How DNA polymerases catalyze DNA replication, repair, and mutation. Biochem. 53 (17), 2749-2751 (2014).
- Lewis, J. S. et al. Tunability of DNA polymerase stability during eukaryotic DNA replication. Mol Cell. 77 (1), 17-25 (2020).
- Chilkova, O. et al. The eukaryotic leading and lagging strand DNA polymerases are loaded onto primer-ends via separate mechanisms but have comparable processivity in the presence of PCNA. Nucleic Acids Res. 35 (19), 6588-6597 (2007).
- Kawasaki, Y. Sugino, A. Yeast replicative DNA polymerases and their role at the replication fork. Mol Cells. 12 (3), 277-285 (2001).
- Stillman, B. DNA polymerases at the replication fork in eukaryotes. Mol Cell. 30 (3), 259-260 (2008).
- Wood, R. D. Shivji, M. K. Which DNA polymerases are used for DNA repair in eukaryotes? Carcinogenesis. 18 (4), 605-610 (1997).
- Budd, M. E. Campbell, J. L. The roles of the eukaryotic DNA polymerases in DNA repair synthesis. Mutat Res DNA Repair. 384 (3), 157-167 (1997).
- Yang, W. Gao, Y. Translesion and repair DNA polymerases: Diverse structure and mechanism. Annu Rev Biochem. 87, 239-261 (2018).
- Bridges, B. A. DNA repair: Polymerases for passing lesions. Curr Biol. 9 (13), 475-477 (1999).
- Wilkinson, E. M., Spenkelink, L. M., Van Oijen, A. M. Observing protein dynamics during DNA-lesion bypass by the replisome. Front Mol Biosci. 9, 968424 (2022).
- Mueller, S. H., Spenkelink, L. M., Van Oijen, A. M. When proteins play tag: The dynamic nature of the replisome. Biophys Rev. 11 (4), 641-651 (2019).
- Zybailov, B. L., Sherpa, M. D., Glazko, G. V., Raney, K. D., Glazko, V. I. G4-quadruplexes, and genome instability. Mol Biol. 47 (2), 197-204 (2013).
- Loeb, L. A. Monnat, R. J. DNA polymerases and human disease. Nat Rev Genet. 9 (8), 594-604 (2008).
- Oganesian, L. Bryan, T. M. Physiological relevance of telomeric G-quadruplex formation: A potential drug target. Bioessays. 29 (2), 155-165 (2007).
- Chambers, V. S. et al. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat Biotechnol. 33 (8), 877-881 (2015).
- Cheng, Y., Zhang, Y., You, H. Characterization of G-quadruplexes folding/unfolding dynamics and interactions with proteins from single-molecule force spectroscopy. Biomol. 11 (11), 1579 (2021).
- Tippana, R., Xiao, W., Myong, S. G-quadruplex conformation and dynamics are determined by loop length and sequence. Nucleic Acids Res. 42 (12), 8106-8114 (2014).
- Kusi-Appauh, N., Ralph, S. F., Van Oijen, A. M., Spenkelink, L. M. Understanding G-quadruplex biology and stability using single-molecule techniques. J Phys Chem B. 127 (25), 5521-5540 (2023).
- Obi, I. et al. Stabilization of G-quadruplex DNA structures in Schizosaccharomyces pombe causes single-strand DNA lesions and impedes DNA replication. Nucleic Acids Res. 48 (19), 10998-11015 (2020).
- Kamath-Loeb, A. S., Loeb, L. A., Johansson, E., Burgers, P. M., Fry, M. Interactions between the Werner syndrome helicase and DNA polymerase delta specifically facilitate copying of tetraplex and hairpin structures of the d(CGG)n trinucleotide repeat sequence. J Biol Chem. 276 (19), 16439-16446 (2001).
- Kaguni, L. S. Clayton, D. A. Template-directed pausing in in vitro DNA synthesis by DNA polymerase a from Drosophila melanogaster embryos. Proc Natl Acad Sci. 79 (4), 983-987 (1982).
- Sato, K., Martin-Pintado, N., Post, H., Altelaar, M., Knipscheer, P. Multistep mechanism of G-quadruplex resolution during DNA replication. Sci Adv. 7 (39), eabf8653 (2021).
- Castillo Bosch, P. et al. FANCJ promotes DNA synthesis through G-quadruplex structures. EMBO J. 33 (21), 2521-2533 (2014).
- Paeschke, K., Capra, John A., Zakian, Virginia A. DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell. 145 (5), 678-691 (2011).
- Guo, L. et al. Joint efforts of replicative helicase and SSB ensure inherent replicative tolerance of G-quadruplex. Adv Sci. (Weinh.). 11 (9), e2307696 (2024).
- Pham, S. Q. T. et al. A new class of quadruplex DNA-binding nickel Schiff base complexes. Dalton Trans. 49 (15), 4843-4860 (2020).
- Georgescu, R. E. et al. Mechanism of asymmetric polymerase assembly at the eukaryotic replication fork. Nat Struct Mol Biol. 21 (8), 664-670 (2014).
- Au - Desjardins, P., Au - Hansen, J. B., Au - Allen, M. Microvolume protein concentration determination using the NanoDrop 2000c spectrophotometer. J Vis Exp. 33, e1610 (2009).
- Summer, H., Grämer, R., Dröge, P. Denaturing urea polyacrylamide gel electrophoresis (Urea PAGE). J Vis Exp. 32, e1485 (2009).
- Geertsema, H. J., Duderstadt, K. E., Van Oijen, A. M. in DNA Replication: Methods and Protocols., eds Vengrova, S., Dalgaard, J.) 219-238, Springer New York, New York, NY (2015).
- Lewis, J. S. et al. Single-molecule visualization of fast polymerase turnover in the bacterial replisome. eLife. 6, e23932 (2017).
- Fish, K. N. Total internal reflection fluorescence (TIRF) microscopy. Curr Protoc Cytom. 12, 12-18 (2009).
- Sommers, J. A., Estep, K. N., Maul, R. W., Brosh, R. M. Biochemical analysis of DNA synthesis blockage by G-quadruplex structure and bypass facilitated by a G4-resolving helicase. Methods. 204, 207-214 (2022).
- Edwards, D. N., Machwe, A., Wang, Z., Orren, D. K. Intramolecular telomeric G-quadruplexes dramatically inhibit DNA synthesis by replicative and translesion polymerases, revealing their potential to lead to genetic change. PLoS One. 9 (1), 80664 (2014).
- Mueller, S. H. et al. Rapid single-molecule characterization of enzymes involved in nucleic-acid metabolism. Nucleic Acids Res. 51 (1), 5 (2022).
- Lewis, J. S., Van Oijen, A. M., Spenkelink, L. M. Embracing heterogeneity: Challenging the paradigm of replisomes as deterministic machines. Chem Rev. 123 (23), 13419-13440 (2023).
- Stingele, J., Bellelli, R., Boulton, S. J. Mechanisms of DNA-protein crosslink repair. Nat Rev Mol Cell Biol. 18 (9), 563-573 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved