Calculating pH for Titration Solutions: Strong Acid/Strong Base
A titration is carried out for 25.00 mL of 0.100 M HCl (strong acid) with 0.100 M of a strong base NaOH. The pH at different volumes of added base solution can be calculated as follows:
(a) Titrant volume = 0 mL. The solution pH is due to the acid ionization of HCl. Because this is a strong acid, the ionization is complete and the hydronium ion molarity is 0.100 M. The pH of the solution is then:

(b) Titrant volume = 12.50 mL. Since the acid sample and the base titrant are both monoprotic and equally concentrated, this titrant addition involves less than a stoichiometric amount of base, and so it is completely consumed by reaction with the excess acid in the sample. The concentration of acid remaining is computed by subtracting the consumed amount from the initial amount and then dividing by the solution volume:
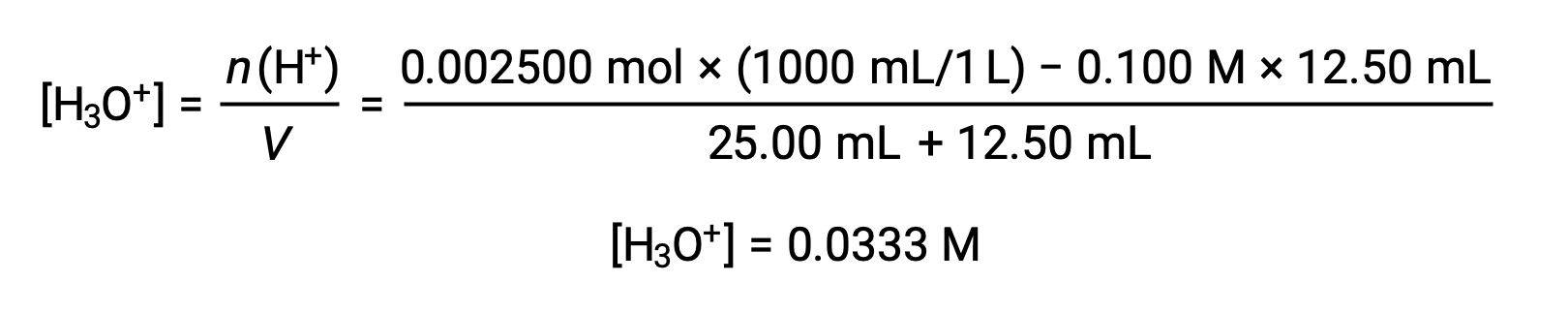
(c) Titrant volume = 25.00 mL. This titrant addition involves a stoichiometric amount of base (the equivalence point), and so only products of the neutralization reaction are in solution (water and NaCl). Neither the cation nor the anion of this salt undergo acid-base ionization; the only process generating hydronium ions is the autoprotolysis of water. The solution is neutral, having a pH = 7.00.
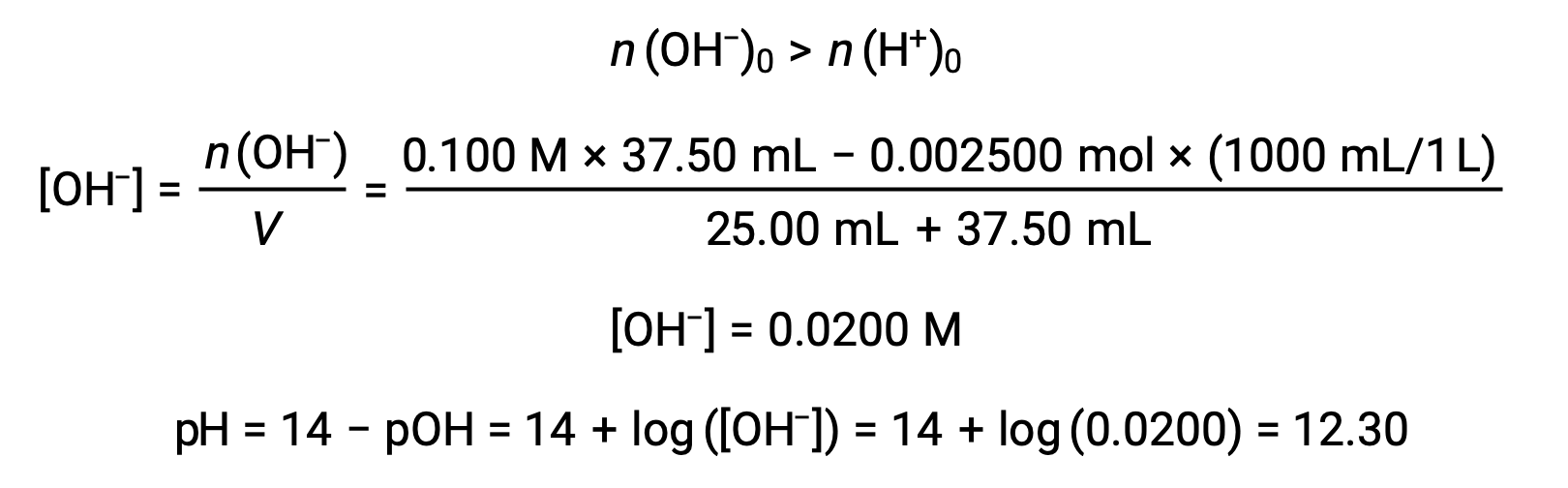
(d) Titrant volume = 37.50 mL. This involves the addition of titrant in excess of the equivalence point. The solution pH is then calculated using the concentration of hydroxide ion:
This text is adapted from Openstax, Chemistry 2e, Section 14.7: Acid-base Titrations.
From Chapter 16:

Now Playing
16.6 : Titration Calculations: Strong Acid - Strong Base
Acid-base and Solubility Equilibria
28.5K Views

16.1 : Common Ion Effect
Acid-base and Solubility Equilibria
40.4K Views

16.2 : Buffers
Acid-base and Solubility Equilibria
162.7K Views

16.3 : Henderson-Hasselbalch Equation
Acid-base and Solubility Equilibria
67.4K Views

16.4 : Calculating pH Changes in a Buffer Solution
Acid-base and Solubility Equilibria
52.0K Views

16.5 : Buffer Effectiveness
Acid-base and Solubility Equilibria
48.0K Views

16.7 : Titration Calculations: Weak Acid - Strong Base
Acid-base and Solubility Equilibria
42.9K Views

16.8 : Indicators
Acid-base and Solubility Equilibria
47.4K Views

16.9 : Titration of a Polyprotic Acid
Acid-base and Solubility Equilibria
95.2K Views

16.10 : Solubility Equilibria
Acid-base and Solubility Equilibria
50.5K Views

16.11 : Factors Affecting Solubility
Acid-base and Solubility Equilibria
32.7K Views

16.12 : Formation of Complex Ions
Acid-base and Solubility Equilibria
22.8K Views

16.13 : Precipitation of Ions
Acid-base and Solubility Equilibria
27.2K Views

16.14 : Qualitative Analysis
Acid-base and Solubility Equilibria
19.6K Views

16.15 : Acid-Base Titration Curves
Acid-base and Solubility Equilibria
124.5K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved