A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Experimental Generation of Carcinoma-Associated Fibroblasts (CAFs) from Human Mammary Fibroblasts
In This Article
Summary
Carcinoma-associated fibroblasts (CAFs) rich in myofibroblasts present within the tumour stroma, play a major role in driving tumour progression. We developed a coimplantation tumour xengraft model for experimentally generating CAFs from human mammary fibroblasts. The protocol describes how to establish CAF myofibroblasts that acquire an ability to promote tumourigenesis.
Abstract
Carcinomas are complex tissues comprised of neoplastic cells and a non-cancerous compartment referred to as the 'stroma'. The stroma consists of extracellular matrix (ECM) and a variety of mesenchymal cells, including fibroblasts, myofibroblasts, endothelial cells, pericytes and leukocytes 1-3.
The tumour-associated stroma is responsive to substantial paracrine signals released by neighbouring carcinoma cells. During the disease process, the stroma often becomes populated by carcinoma-associated fibroblasts (CAFs) including large numbers of myofibroblasts. These cells have previously been extracted from many different types of human carcinomas for their in vitro culture. A subpopulation of CAFs is distinguishable through their up-regulation of α-smooth muscle actin (α-SMA) expression4,5. These cells are a hallmark of 'activated fibroblasts' that share similar properties with myofibroblasts commonly observed in injured and fibrotic tissues 6. The presence of this myofibroblastic CAF subset is highly related to high-grade malignancies and associated with poor prognoses in patients.
Many laboratories, including our own, have shown that CAFs, when injected with carcinoma cells into immunodeficient mice, are capable of substantially promoting tumourigenesis 7-10. CAFs prepared from carcinoma patients, however, frequently undergo senescence during propagation in culture limiting the extensiveness of their use throughout ongoing experimentation. To overcome this difficulty, we developed a novel technique to experimentally generate immortalised human mammary CAF cell lines (exp-CAFs) from human mammary fibroblasts, using a coimplantation breast tumour xenograft model.
In order to generate exp-CAFs, parental human mammary fibroblasts, obtained from the reduction mammoplasty tissue, were first immortalised with hTERT, the catalytic subunit of the telomerase holoenzyme, and engineered to express GFP and a puromycin resistance gene. These cells were coimplanted with MCF-7 human breast carcinoma cells expressing an activated ras oncogene (MCF-7-ras cells) into a mouse xenograft. After a period of incubation in vivo, the initially injected human mammary fibroblasts were extracted from the tumour xenografts on the basis of their puromycin resistance 11.
We observed that the resident human mammary fibroblasts have differentiated, adopting a myofibroblastic phenotype and acquired tumour-promoting properties during the course of tumour progression. Importantly, these cells, defined as exp-CAFs, closely mimic the tumour-promoting myofibroblastic phenotype of CAFs isolated from breast carcinomas dissected from patients. Our tumour xenograft-derived exp-CAFs therefore provide an effective model to study the biology of CAFs in human breast carcinomas. The described protocol may also be extended for generating and characterising various CAF populations derived from other types of human carcinomas.
Protocol
1. Isolation of primary cultured human normal mammary fibroblasts
Experimental procedures for isolating primary cultured human normal mammary fibroblasts are outlined in Fig. 1A.
- Prepare the cell dissociation buffer, as described previously12: Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal calf serum (FCS), penicillin-streptomycin (200 units/ml), collagenase type I (1 mg/ml) and hyaluronidase (125 units/ml).
- Wash the breast tissue dissected from a reduction mammoplasty (~0.5 gram) several times in phosphate buffered saline (PBS). Mince the tissue into small fragments (<1.5 mm3) using sterile razor blades.
- Transfer the tissue fragments into a 15 ml conical tube containing an appropriate amount of the above cell dissociation buffer (10 ml per 0.5 gram of tissue) and vortex for 1 min at maximum speed.
- Digest the tissue fragments in the cell dissociation buffer for 12-18 h at 37°C with slow agitation. *Note that there will be still many pieces of undigested tissue fragments remaining in the tube.
- Incubate the tube for 5 min at room temperature without agitation. Transfer the resulting stromal cell-enriched supernatant into a new conical tube using a 5 ml serological pipet.
- Centrifuge the stromal fraction for 5 min at 250 X g and resuspend the cell pellet in PBS. Centrifuge again for 5 min at 250 X g and resuspend the cell pellet in DMEM supplemented with 10% FCS. Culture the cells in DMEM containing 10% FCS in a 15 cm petri dish at 37°C and 5% carbon dioxide.
- Propagate the cells until confluent. It will usually take 8-10 days. Store the cells at -80°C using freezing medium (10 % dimethyl sulfoxide and 20% FCS in DMEM). Prepare 5 frozen vials as an original stock. Thaw one vial and expand the cells to prepare 5 vials for the secondary stock containing fibroblasts passaged within 5 population doublings (PDs) for the subsequent experiments. These procedures minimise clonal selection and culture stress that could occur during extended tissue culture. *Note that P1.1-7 can also be used for isolating CAFs from breast carcinomas obtained by a mastectomy12.
2. Generation of GFP-labelled, puromycin-resistant, immortalised human normal mammary fibroblasts
- To immortalise the primary human normal mammary fibroblasts isolated in P1.7), introduce a retroviral pMIG (MSCV-IRES-GFP) vector12, expressing both hTERT and GFP. Culture the cells for 4-5 days and then sort the GFP-positive cells using flow cytometry.
- Introduce a retroviral pBabe-puro construct encoding a puromycin resistance gene into the GFP-labelled immortalised fibroblasts. Culture the cells for 5-7 days in the presence of puromycin (final concentration: 1 μg/ml) to isolate the GFP-positive (GFP+), puromycin-resistant (puroR) immortalised human mammary fibroblasts.
- Next, to examine whether these fibroblasts produce active viruses, which can lead to the horizontal transfer of genes encoding GFP and puromycin resistance, grow 2.5x105 GFP+puroR immortalized human mammary fibroblasts in a 6 cm Petri dish for 2 days.
- Filter medium conditioned by these fibroblasts using a syringe filter (pore size: 0.45 μm) and add it onto 10T1/2 mouse fibroblastic cells for 12h in the presence of protamine sulfate (5 μg/ml) which increases efficiency of the virus infection. The medium is then changed to 10% FCS-DMEM for additional 2 days.
- Examine the cells by a fluorescence microscopy. 10T1/2 cells infected by GFP virus will become GFP-positive. Treat also the cells with puromycin (1 μg/ml) for 5-7 days and observe the plate if any puroR 10T1/2 cells are present and form colonies after the puromycin treatment.
- Note that the horizontal gene transfer by active viruses produced by the parental GFP+puroR human mammary fibroblasts might make the surrounding murine cells and/or carcinoma cells GFP+puroR within tumour xenografts. This may hinder the processes of isolating the initially injected GFP+puroR human mammary fibroblasts from the tumour xenografts.
3a. Coinjection of human mammary fibroblasts with breast carcinoma cells into an immunodeficient mouse
Experimental procedures for generating exp-CAFs are illustrated in Fig. 1Ba.
- Mix 1x106 MCF-7-ras human breast carcinoma cells and 3x106 GFP+puroR, immortalised human mammary fibroblasts generated in P2.2). Prepare the cells in 400 μl of culture medium with 50% (v/v) Matrigel per injection.
- Inject the cell mixture subcutaneously into an immunodeficient nude mouse. When CAFs are extracted independently from different tumours, implant each mixture into right and left flanks of several mice.
3b. Injection of human mammary fibroblasts into an immunodeficient mouse
- To generate control fibroblasts against exp-CAFs, prepare 3x106 GFP+puroR immortalised human mammary fibroblasts generated in P2.2 in 400 μl of culture medium with 50% (v/v) Matrigel per injection (Fig. 1Bb). DO NOT mix the fibroblasts with carcinoma cells.
- Implant the fibroblasts prepared in P3b.1 into subcutaneous sites of nude mice.
*Note that the inoculated fibroblasts will form the fibroblastic tissue but not tumour under the skin.
4a. Dissection of tumour xenograft
- Euthanise the mouse harbouring a subcutaneous human breast cancer xenograft 42 days post-implantation (Fig. 1Ba). *Note that when other carcinoma cells and/or fibroblasts are coimplanted, fibroblasts may need to be incubated for longer than 42 days within the tumour xenograft to initiate their myofibroblastic phenotype.
- Harvest the tumour xenograft from the flank of the mouse by blunt dissection using forceps and scissors. Immerse the tumour tissue in PBS.
4b. Dissection of fibroblast xenograft
- Euthanise the mouse harbouring the fibroblast xenograft 42 days post-implantation (Fig. 1Bb).
- Dissect the subcutaneous fibroblast xenograft using forceps and scissors. Place the fibroblastic tissue into PBS. *Note that a fibroblast xenograft, which appears as a tiny transparent tissue, may be difficult to find under the skin.
5. Preparation of primary cultured cells from xenografts
- Use a sterile biosafety cabinet to dissect the tumour (˜0.5 gram) or fibroblastic tissue (˜0.1 gram). Transfer the excised tissues onto the top of a 15 cm culture dish with 1 ml of PBS. Mince the tissues into small tissue fragments (<1.5 mm3) using sterile razor blades. *Note that if the fibroblast xenograft weighs less than 0.1 gram, collect a few additional fibroblast xenografts and mix them together to increase the number of cells for isolation.
- Transfer the tissue fragments into a 15 ml conical tube containing the cell dissociation buffer (10 ml per 0.5 gram of tissue) and vortex for 1 min at maximum speed.
- Incubate the cell suspension for 3 h at 37°C with continuous slow agitation.
6. Isolation of puromycin-resistant cells in culture
- Centrifuge the cell suspension dissociated either from tumour or fibroblast xenograft for 5 min at 250 X g and resuspend the cell pellet in PBS. Centrifuge again for 5 min at 250 X g. Resuspend the resulting cell pellet in 10% FCS-DMEM. Culture the cells in a 15 cm petri dish in the presence of puromycin (1 μg/ml) at 37°C and 5% carbon dioxide in order to eliminate any contaminating carcinoma cells and/or murine stromal cells. Propagate the cells until confluent (2-4 weeks). Store the cells at -80°C using freezing medium. *Note that the resulting puroR cells, designated 42-day-old exp-CAF1 (Fig. 1Ba) or control fibroblast-1 cells (Fig. 1Bb), are immortal and positive for GFP. It is recommended to check if these cells produce active viruses using 10T1/2 cells, as described in P2.3-6.
7a. Isolation of exp-CAF2 cells in culture
To further boost the activated myofibroblastic phenotype of CAFs, mix the 42-day-old exp-CAF1 cells with MCF-7-ras cells and inject them again subcutaneously into a nude mouse for additional periods of 200 days. Dissect and dissociate the tumour xenograft into a single-cell suspension and culture the cells in a 15 cm petri dish with 10% FCS-DMEM in the presence of puromycin (1 μg/ml), as indicated in P6.1. Propagate the cells until confluent (8-10 days). Store the cells at -80°C using freezing medium. The resulting puroR cells are named 242-day-old exp-CAF2 cells (Fig. 1Ba).
7b. Extraction of control fibroblast-2 cells in culture
To generate control fibroblasts against exp-CAF2 cells, inject 42-day-old control fibroblast-1 cells alone without carcinoma cells subcutaneously into a nude mouse additionally for 200 days. Dissect and dissociate the fibroblastic tissue into a single cell suspension and culture the cells in a 15 cm petri dish with 10% FCS-DMEM in the presence of puromycin (1 μg/ml) as indicated in P6.1. Propagate the cells until confluent (3-4 weeks). Store the cells at -80°C using freezing medium. The resulting puroR cells are named control fibroblast-2 cells (Fig. 1Bb).
8. Representative Results:
Control fibroblast-2 and exp-CAF2 cells, which have been extracted from breast tumour xenografts, stained strongly positive for mesenchymal markers, including human-specific vimentin, prolyl-4-hydroxylase, collagen 1A, fibronectin, S100A4, and fibroblast surface protein (Fig. 2A)11, indicating human origin and mesenchymal nature of these cells. In contrast, the cytokeratin, a marker for epithelial cells, was not stained in these GFP+fibroblasts (Fig 2B). These findings therefore suggest that the extracted exp-CAF2 and control fibroblast-2 cells have originated from the parental human mammary fibroblasts initially introduced into mouse xenografts.
Importantly, a higher proportion of exp-CAF2 cells stained positive for α-SMA and extracellular matrix glycoprotein tenascin-C5, both of which are markers of myofibroblasts compared to 42-day-old exp-CAF1 and control fibroblast-2 cells (Fig. 2C, D)11. These data indicate that resident human mammary fibroblasts progressively evolve into CAF myofibroblasts within tumour xenografts.

Figure 1 Schematic representation of isolation of human mammary fibroblasts. A) The reduction mammoplasty tissue was minced using sterile razor blades (P1.2) and transferred into a 15 ml conical tube (P1.3). The small tissue fragments were digested in the cell dissociation buffer (P1.4) and prepared for culture in vitro (P1.5-7). To immortalise the isolated primary human mammary fibroblasts, a retroviral pMIG (MSCV-IRES-GFP) vector, expressing both hTERT and GFP, was introduced, and the resulting GFP-positive cells were sorted using flow cytometry (P2.1). A retroviral pBabe-puro vector encoding a puromycin resistance gene was then introduced into these cells. Upon the puromycin treatment, GFP-labelled (GFP+) puromycin-resistant (PuroR), immortalised human mammary fibroblasts were isolated (P2.2).
Ba) To generate exp-CAFs, GFP+puroR immortalised human mammary fibroblasts were coinjected with MCF-7-ras breast carcinoma cells subcutaneously into an immunodeficient nude mouse (P3a). The tumour xenograft was resected at 42 days after implantation (P4a) and dissociated into a single-cell suspension (P5). These cells were then cultured in vitro in the presence of puromycin to eliminate any contaminating carcinoma cells and mouse stromal cells (P6). The resulting puromycin-resistant cells were termed experimentally generated CAF1 (exp-CAF1) cells. These cells, resected 42 days post-implantation, were once again mixed with MCF-7-ras cells and implanted subcutaneously into a host mouse as before (P7a). The resulting tumour was allowed to grow for additional periods of 200 days, then dissected, dissociated, and cultured in the presence of puromycin. The isolated puromycin-resistant cells were termed exp-CAF2 cells (242-day-old).
Bb)To isolate control cells against exp-CAFs, GFP+puroR immortalized human mammary fibroblasts were injected subcutaneously into a nude mouse as pure cultures without MCF-7-ras cells (P3b). The fibroblastic tissue, dissected 42 days post-implantation (P4b), was dissociated into single-cell suspensions (P5) and puromycin-resistant cells, named control fibroblast-1 cells, were isolated as described earlier (P6). These fibroblasts were once again implanted subcutaneously into a nude mouse without MCF-7-ras cells additionally for 200 days (P7b). The fibroblastic tissue was dissected, dissociated, and cultured in the presence of puromycin. The isolated puromycin-resistant cells were termed control fibroblast-2 cells (242-day-old).
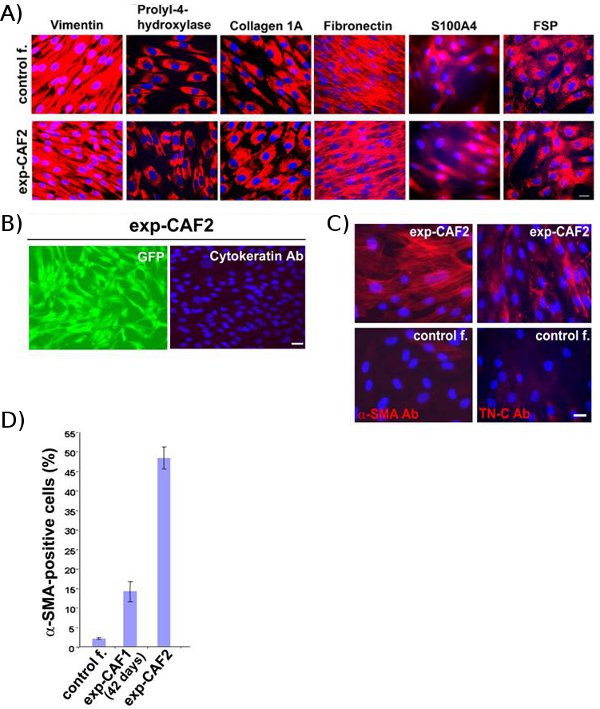
Figure 2 Exp-CAFs and the control fibroblasts originate from the parental human mammary fibroblasts. (A) Immunofluorescence analyses of control fibroblast-2 (control f.) and exp-CAF2 cells. The both cell types stain positive for mesenchymal markers (red), including human vimentin, prolyl-4-hydroxylase, collagen 1A, fibronectin, S100A4, and fibroblast surface protein. (B) In contrast, pan-cytokeratin, a marker for epithelial cells, is not detected in exp-CAF2 cells expressing GFP (green). Cell nuclei are stained with 4'-6-Diamidino-2-phenylindole (DAPI) (blue). Scale bar, 50 μm (referred from Kojima et al.11)
(C) Immunofluorescence of exp-CAF2 cells and control fibroblast-2 cells (control f.) using antibodies against α-SMA (red) or tenascin-C (TN-C) (red). Cell nuclei are stained with DAPI (blue). Scale bar, 50 μm. (D) 48% of exp-CAF2 cells stain positive for α-SMA, whereas 14% of 42-day-old exp-CAF1 and 2.5% of the control fibroblast-2 cell populations are positive for α-SMA. (referred from Kojima et al.11)
Discussion
The lack of CAF-specific markers and the level of heterogeneity observed amongst CAFs render the characterisation of this cell type a challenge in itself. Studying CAFs in vitro has also been hindered by the additional complication that these cells senesce and stop proliferating when cultured for a long period. Our previous attempt to directly immortalise primary CAFs using a retroviral hTERT cDNA construct was unsuccessful. Therefore, to further investigate the tumour promoting properties of these cells, we dev...
Disclosures
No conflicts of interest declared.
Acknowledgements
We thank Dr. Robert A. Weinberg (Whitehead Institute for Biomedical Research, Cambridge) for generous support and supervision of this work and Mr. Kieran Mellody (University of Manchester, Manchester) for critical editing of this manuscript. This project was supported by Research UK (CR-UK) grant number C147/A6058 (A.O.).
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
| DMEM | Invitrogen | 61965-026 | ||
| Fetal calf serum | GIBCO | 10270 | ||
| Penicillin-streptomycin | Invitrogen | 15140-122 | ||
| Collagenase type I | Sigma | C0130-1G | ||
| hyaluronidase | Sigma | H4272 | ||
| Vimentin (V9) antibody | Novocastra Laboratories | NCL-L- VIM-V9 | ||
| Tenascin C (BC-8) antibody | a gift from | |||
| α-SMA-Cy3 (1A4) antibody | Sigma | C6198 | ||
Prolyl-4-hydroxylase | Dako | M0877 | ||
| (5B5) antibody | ||||
| Collagen type1 1A antibody | Sigma | HPA011795 | ||
| Pan-cytokeratin antibody | Sigma | C5992 | ||
| Fibronectin antibody | BD Biosciences | 610077 | ||
S100A4/FSP-1 (fibroblast- specific protein-1) antibody | Dako | A5114 | ||
Fibroblast surface protein (clone 1B10) antibody | Abcam | ab11333 | ||
| MSCV-IRES-GFP construct | Request to the the authors | |||
| pBabe-puro construct | Purchase from Addgene | |||
| Puromycin | Sigma | P8833 | ||
| DAPI | Sigma | D9564 | ||
| 15 ml conical tube | Corning | 430766 | ||
| Nude mouse | Taconic | NCRNU-F | Female NCr nude | |
| C3H/10T1/2 cells | ATCC | CCL-226 |
References
- Ronnov-Jessen, L., Bissell, M. J. Breast cancer by proxy: can the microenvironment be both the cause and consequence?. Trends Mol. Med. 15, 5-13 (2009).
- Mueller, M. M., Fusenig, N. E. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer. 4, 839-849 (2004).
- Bhowmick, N. A., Neilson, E. G., Moses, H. L. Stromal fibroblasts in cancer initiation and progression. Nature. 432, 332-337 (2004).
- Kalluri, R., Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer. 6, 392-401 (2006).
- De Wever, O. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. 18, 1016-1018 (2004).
- Serini, G., Gabbiani, G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp. Cell. Res. 250, 273-283 (1999).
- Shimoda, M., Mellody, K. T., Orimo, A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin. Cell. Dev. Biol. 21, 19-25 (2010).
- Pietras, K., Ostman, A. Hallmarks of cancer: interactions with the tumor stroma. Exp. Cell. Res. 316, 1324-1331 (2010).
- Polyak, K., Haviv, I., Campbell, I. G. Co-evolution of tumor cells and their microenvironment. Trends in Genetics. 25, 30-38 (2009).
- Franco, O. E., Shaw, A. K., Strand, D. W., Hayward, S. W. Cancer associated fibroblasts in cancer pathogenesis. Semin. Cell. Dev. Biol. 21, 33-39 (2010).
- Kojima, Y. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc. Natl. Acad. Sci. U. S. A. 107, 20009-20014 (2010).
- Orimo, A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 121, 335-348 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved