A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Development of a Unilaterally-lesioned 6-OHDA Mouse Model of Parkinson's Disease
In This Article
Summary
A protocol for performing unilateral 6-OHDA lesions of the medial forebrain bundle in mice is described. This method has a low mortality rate (13.3 %) with 89% of the surviving animals showing >95% loss of striatal dopamine and 90.63±-4.02 % ipsiversive rotational bias towards the side of the lesion.
Abstract
The unilaterally lesioned 6-hyroxydopamine (6-OHDA)-lesioned rat model of Parkinson's disease (PD) has proved to be invaluable in advancing our understanding of the mechanisms underlying parkinsonian symptoms, since it recapitulates the changes in basal ganglia circuitry and pharmacology observed in parkinsonian patients1-4. However, the precise cellular and molecular changes occurring at cortico-striatal synapses of the output pathways within the striatum, which is the major input region of the basal ganglia remain elusive, and this is believed to be site where pathological abnormalities underlying parkinsonian symptoms arise3,5.
In PD, understanding the mechanisms underlying changes in basal ganglia circuitry following degeneration of the nigro-striatal pathway has been greatly advanced by the development of bacterial artificial chromosome (BAC) mice over-expressing green fluorescent proteins driven by promoters specific for the two striatal output pathways (direct pathway: eGFP-D1; indirect pathway: eGFP-D2 and eGFP-A2a)8, allowing them to be studied in isolation. For example, recent studies have suggested that there are pathological changes in synaptic plasticity in parkinsonian mice9,10. However, these studies utilised juvenile mice and acute models of parkinsonism. It is unclear whether the changes described in adult rats with stable 6-OHDA lesions also occur in these models. Other groups have attempted to generate a stable unilaterally-lesioned 6-OHDA adult mouse model of PD by lesioning the medial forebrain bundle (MFB), unfortunately, the mortality rate in this study was extremely high, with only 14% surviving the surgery for 21 days or longer11. More recent studies have generated intra-nigral lesions with both a low mortality rate >80% loss of dopaminergic neurons, however expression of L-DOPA induced dyskinesia11,12,13,14 was variable in these studies. Another well established mouse model of PD is the MPTP-lesioned mouse15. Whilst this model has proven useful in the assessment of potential neuroprotective agents16, it is less suitable for understanding mechanisms underlying symptoms of PD, as this model often fails to induce motor deficits, and shows a wide variability in the extent of lesion17, 18.
Here we have developed a stable unilateral 6-OHDA-lesioned mouse model of PD by direct administration of 6-OHDA into the MFB, which consistently causes >95% loss of striatal dopamine (as measured by HPLC), as well as producing the behavioural imbalances observed in the well characterised unilateral 6-OHDA-lesioned rat model of PD. This newly developed mouse model of PD will prove a valuable tool in understanding the mechanisms underlying generation of parkinsonian symptoms.
Protocol
1. Housing and preparation of mice
- Maintain a colony of bacterial artificial chromosome (BAC) driven transgenic mice8 (Mutant Mouse Regional Resource Center (MMRRC) FVB in a 12:12 h light-dark cycle with free access to food and water. These mice are pure FVB mice, and it is not necessary to cross these mice with any other strain, either for breeding purposes, or to ensure success of the 6-OHDA lesion procedure.
- In order to generate a parkinsonian model, adult mice aged postnatal day 31-42 (P31-42) are required for 6-OHDA-lesion and sham surgeries.
- To reduce the risk of infection, administer Baytril (antibiotic) in the drinking water for 24 hours prior to surgery.
2. Preparation of drugs for surgery
A premedication of desipramine and pargyline is usually administered to rodents prior to injection of 6-hydroxydopamine (6-OHDA) to increase the selectivity and efficacy of 6-OHDA-induced lesions. The noradrenaline / 5HT uptake inhibitor, desipramine decreases 6-OHDA-induced noradrenaline and 5HT depletion22, whereas the monoamine oxidase inhibitor, pargyline enhances the sensitivity of dopaminergic terminals to 6-OHDA, by reducing extrasynaptic breakdown of 6-hydroxydopamine31.
- The premedication (desipramine hydrochloride (HCl) and pargyline HCl) can be made in large volumes and stored at -80°C until use. Weigh out the appropriate amount of desipramine HCl to supply each mouse with 25 mg/kg for the number of animals that will receive surgery on that day. The low weight of the mouse makes it challenging to deliver small volumes accurately; therefore prepare desipramine hydrochloride at a concentration of 2.5 mg/ml and administered to the animal at 10 ml/kg. The weight of the salt component of the compound must be accounted for such that the correct concentration is achieved. Correction for the weight of the HCl salt in desipramine hydrochloride: Molecular weight (MW) of desipramine HCl: 302.84 MW HCl: 36.46 MW of free base: 266.38 Correction factor: 302.84 / 266.38 = 1.137 For 10 ml of solution: = 2.5mg/ml desipramine x 10.0ml x Correction factor = 2.5mg/ml x 10.0ml x 1.137 = 28.43mg of desipramine hydrochloride
- Weigh out the appropriate amount of pargyline HCl to supply each mouse with 5mg/kg for the number of animals receiving surgery that day. Correct for the salt component of the compound as described above. The low weight of the mouse makes it challenging to deliver small volumes accurately; therefore prepare pargyline hydrochloride at a concentration of 0.5 mg/ml and administer to the animal at 10 ml/kg.
Correct for the weight of the HCl salt in pargyline hydrochloride:
MW of pargyline HCl: 195.69 MW HCl: 36.46
MW of free base: 159.23 Correction factor: 195.69 / 159.23 = 1.229
For 10 ml of solution weigh out:
= 0.5 mg/ml pargyline x 10.0 ml x Correction factor
= 0.5 mg/ml x 10.0 ml x 1.229
= 6.15mg of pargyline hydrochloride - Combine 28.43mg desipramine HCl and 6.15mg pargyline HCl into a 10 ml glass graduated cylinder, and add 8 ml sterile saline (0.9%). Vortex and heat to 45°C until the mixture is dissolved. The pH of the solution will now be around 3, which if administered to the mice will cause discomfort and post-operative complications involving urinary and gastrointestinal tract function. Add drops of NaOH (1M) until the pH is 7.4. Top up the volume to 10ml with sterile saline (0.9%), and vortex. Label, date and freeze at ~80°C. Given that pargyline HCl is not soluble in saline at room temperature, throughout the surgical period, store the premedication mixture in a water bath heated to 37°C in the operating room.
- Solutions of 6-OHDA must be prepared immediately prior to surgeries. The vehicle used to dissolve 6-OHDA hydrobromide (6-OHDA.Br) solution is sterile saline (0.9%) solution containing ascorbic acid (0.2%). Ascorbic acid is needed to stabilize 6-OHDA.Br, as it prevents oxidation of 6-OHDA.Br to an inactive form. Weigh out 0.2 g of ascorbic acid, then add to an empty 1 L graduated cylinder, top up to 1 L with sterile saline (0.9%) and stir. Label, date, and store at ~80°C until required.
- Weigh out the appropriate amount of 6-OHDA.HBr to make a 15.0 mg/ml solution to supply each mouse with 3.0 μg. Since 6-OHDA.Br is light and heat sensitive avoid exposure to light, and place on ice prior to weighing.
Correct for the weight of the HBr salt in 6-OHDA.HBr:
MW of 6-OHDA.HBr: 250.09 MW HBr: 79.9 MW of free base: 170.19
Correction factor: 250.09 / 170.19 = 1.47
For 0.5 ml of solution weigh out:
= 15.0 mg/ml 6-OHDA x 0.5 ml x Correction factor
= 15.0 mg/ml 6-OHDA x 0.5 ml x 1.47
= 11.03 mg of 6-OHDA.HBr - Weigh 6-OHDA.HBr into a sterile 1.0 ml tube covered in aluminum foil, and add 0.5 ml vehicle (sterile saline (0.9%), ascorbic acid 0.02% pH 7.4). Vortex until the mixture is dissolved, label, and place immediately on ice until use. Note that the 6-OHDA solution must be used within approximately 6 hours after preparation. If the solution turns dark brown either directly after preparation, or during the surgery, this indicates that 6-OHDA.HBr has become oxidized, and is no longer an effective neurotoxin, so must be discarded and prepared once again.
- For sham-operated animals, make up an equivalent volume of vehicle (sterile saline (0.9%), ascorbic acid 0.02% pH 7.4) to that which the 6-OHDA-lesioned animals will receive, and place in a 1.0 ml sterile tube wrapped in tin foil, label and place immediately on ice until use.
3. Setting up the surgical apparatus
Surgical tools must be sterilised using an autoclave prior to surgery. Between each operation, surgical tools must be sterilised in 95% ethanol followed by heating to 250°C for 2 minutes. All experiments must be performed in accordance with the guidelines of the appropriate Animal Care Committees of the affiliated institution and country.
- Clean all surfaces and equipment with isopropyl alcohol prior to setting up the equipment. Wear sterile gloves when handling clean surgical equipment.
- Set-up a mouse recovery cage with paper towels on the base. Place under a heating lamp or on top of a heated pad.
- Set up the anaesthesia trolley, ensuring that the oxygen inflow is connected and open, and the isoflurane reservoir is completely full. Set the oxygen flow to 1 L/min, and the isoflurane to 2-4%. Ideally there will be two outflow ports from the anaesthetic machine to deliver the oxygen isoflurane mixture to an induction chamber, as well as for maintenance while the animal is in the stereotaxic frame.
- Correct assembly of the infusion apparatus is critical for ensuring successful lesions, as trapped air or blocked needles decreases the volume of 6-OHDA infused at the lesion site. Assemble the infusion apparatus as demonstrated in figure 1. Thread one RN metal nut onto one side of the PEEK tubing (RN compression kit (fitting 1/16th inch)), followed by the PEEK cup ferrule, then the canonical PFA ferrule. Orient the ferrules so that the cone on the canonical PFA ferrule will slip into the mating part of the PEEK cup ferrule. Place into the dual small hub RN coupler (#1), ensuring the connection is tight. On the other end of the dual small hub RN coupler insert the 33 gauge injection needle. Thread the needle through one of the RN metal nuts and tighten. Ensure the 33 gauge needle is at an 180° angle to the dual small hub RN coupler (#1). On the other end of the PEEK tubing, thread one of the RN metal nuts followed by a PEEK ferrule, then the PFA ferrule. Insert metal nut and ferrule assembly into a second dual small hub RN coupler and tighten the connection. On the other end of a second small dual RN hub coupler (#2) insert the luer to small hub RN adaptor (luer adaptor), and tighten the connection.
- Next the infusion apparatus must be primed (see Figure 1). Fill the 250 μl and 1.0 μl Hamilton syringes with sterile water, ensuring there are no air bubbles. Attach the 250 μl syringe to the luer adaptor and depress the plunger of the 250 μl syringe such that water is released through the 33 gauge injection needle on the other end of the tubing. Continue pushing water through the system while pulling the 250 μl syringe out of the luer adaptor, leaving a small water bead on the end of the luer adaptor. Depress the plunger of the 1.0 μl syringe until a small water bead is ejected. Carefully insert the 1.0 μl syringe into the luer adaptor, be sure that the water bead on the 1.0 μl syringe connects with the water bead present on the luer adaptor, and completely insert the 1.0 μl syringe into the luer adaptor. Ensure no bubbles in the small dual RN hub coupler.
- Now that the system is primed, the perfusion apparatus can be secured to the stereotaxic frame and infusion pump. Clamp the small dual RN hub coupler with the 33 gauge injection needle attached to the manipulator arm of the stereotaxic frame, then clamp the 1.0 μl syringe to the perfusion pump, such that when the perfusion pump begins the syringe is pressed against the clamp and cannot move. Program the infusion pump to allow an infusion rate of 0.1 μl/min.
4. Unilateral 6-OHDA lesion surgery
- Thirty minutes prior to operations, weigh each animal and record the weight. Systemically administer desipramine hydrochloride (2.5 mg/ml, Sigma Aldrich) and pargyline hydrochloride (0.5 mg/ml, Sigma Aldrich) (0.9% sterile saline, pH 7.4) at 10 ml/kg by intra-peritoneal injection (i.p) to one mouse using a 27g needle attached to a 1ml syringe. For example, a 30.0 g mouse would receive 300 μl of premedication. Warm up the heating disc and place this under the ear and incisor bar. The heating disc will help to maintain the temperature of the animal during surgery and keep the animal at an ideal height to fit the ear and incisor bars of the stereotaxic frame.
- Fifteen minutes following administration of the desipramine HCl and pargyline HCl solution, place the mouse in a closed anaesthesia chamber, and anesthetise the animal using isoflurane inhalation (2-3% in O2). The animal is sufficiently anesthetised when it shows no response to hind leg pinch and no blink reflex.
- Shave the top of the mouse's head, and apply the topical analgesic lidocaine directly to the skin using cotton wool. Five minutes following lidocaine application, sterilise the head of the animal with betadine solution.
- Place the animal in a stereotaxic frame adapted for mice. Firstly place the animal into the incisor bars (incisor bar setting: -3.0 to +2.0 mm) with the anaesthesia mask placed over the face of the animal. Adjust the oxygen flow to 1 L/min, and the isoflurane to 1.5-2%. The incisor bar should be at a level relative to the ear cups such that the top of the skull is level. Insert the ear cups (diameter 5.0 mm). The ear cups have been inserted correctly when the head is completely flat, and cannot be moved in either direction.
- Cut along the midline of the skin on top of the head of the mouse using a scalpel blade, and retract the skin. Dry the surface of the skull using gauze.
- Push down plunger of the 1.0 μl syringe, and place the 33 gauge injection needle into the 1 ml tube covered in tin foil containing 6-OHDA solution (15 mg/ml). Draw back slowly on the plunger of the 1.0 μl syringe while keeping the 33 gauge injection needle immersed in the 6-OHDA solution (15 mg/ml).
- Create a 1 cm incision along the midline of the skull. Advance the tip of the injection needle towards Bregma, dispense a small amount of 6-OHDA solution from the 33 gauge injection needle to form a small bead. Slowly lower the injection needle tip to Bregma, when the bead touches Bregma stop advancing the tip of the needle and record these coordinates. Retract the injection needle 2 mm in the dorsal direction, and move along the sagittal suture in a rostral caudal direction towards lambda. Advance a small amount of 6-OHDA solution from the 33 gauge injection needle to form a small bead. Slowly lower the injection needle tip to Lambda, when the bead contacts Lambda stop advancing the tip of the needle and record these coordinates. The medial lateral (ML), and dorsal ventral (DV) coordinates should be identical for Bregma and Lambda, if they are not, adjust the incisor bar accordingly for the AP coordinates, and the ear bars for the ML coordinates. Move the needle to co-ordinates AP: -1.2 mm, ML: -1.1 mm relative to Bregma according to the Mouse Brain Atlas in Stereotaxic Co-ordinates25. Retract the needle, and burr a hole into the skull using a 25 gauge needle.
- Return the injection needle to the above co-ordinates, and insert the needle to DV: -5mm. Infuse 0.2 μl of 6-OHDA or vehicle unilaterally into the median forebrain bundle at a rate of 0.1 μl/min (3 μg total). Upon completion of administration of 6-OHDA or vehicle, leave the needle in place for a further five minutes to allow diffusion away from the injection site.
- Slowly retract the needle.
- Close the incision to the scalp using three sutures, and deliver 1ml of Lactated Ringer's solution subcutaneously (s.c).
- Remove the animal from stereotaxic frame, and place in the recovery cage until consciousness is regained.
5. Post-operative care of 6-OHDA-lesioned animals
- Place animals in cages (3 mice/cage) and allow free access to food and sugared water (10 mM). Provide Nutra-gel and KMR (kitten milk replacement) in food containers on the floor of the cage to stimulate appetite and gastrointestinal motility.
- Inspect mice daily post-surgery for 2 weeks24. At each inspection special attention should be paid to the alertness of the animal by assessing the ability of the animal to move around the cage. Food and water intake from the last observation period, as well as the presence and consistency of fecal matter should also be observed. A common post-operative complication is dehydration this is evident by slow retraction of the skin following skin pinch. If this is observed deliver 1ml of Lactated Ringer's solution s.c. for 1 week or until symptoms improve. In the case of male animals, the genitals should be carefully observed for penis prolapse identified by redness of the penis. If this occurs apply mucol gel to the penis of the affected animal, and deliver 1 ml of Lactated Ringer's solution s.c. for 1 week or until symptoms improve.
- Weigh animals daily to monitor changes in body weight.
- In the case where the i.p. injection has caused a small tear in the gastrointestinal system, abdominal infection may result. Therefore, assess for the prsence of infection of the abdomen, evident by swollen distended abdomen. Treat by administering Baytril in the drining water for 3-4 days or until symptoms improve.
6. Parkinsonian assessment
To estimate the extent of the lesion, behavioural assessment was performed 14-21 days following 6-OHDA-lesion surgery, when the amount of dopamine depletion is maximal and stable26.
- Following i.p. injection of 0.01 ml/g 0.9% sterile saline, place mice in glass cylinders (11 cm x 9.5 cm) and record their activity using a videocamera (CG9, Sanyo).
- Watching the video, count the number of complete 360° rotations made in both the ipsiversive and contraversive direction relative to the lesion.
- The greater the bias towards ipsiversive rotations, the more parkinsonian the animal, thus calculate the proportion of ipsiversive rotations as a percentage of net (contraversive and ipsiversive) rotational behaviour.
7. Determination of striatal dopamine content by HPLC
- At least 21 days following the 6-OHDA lesion surgery, and upon completion of the behavioural studies, sacrifice the mice by over-dose of anesthesia, and carefully remove the brain. Retain one slice of striatum (230 μm) from each hemisphere in each mouse, and snap freeze immediately after sectioning and store at -80°C until preparation of striatal samples.
- The HPLC sample buffer (0.1 M PCA with 2 mM glutathione) prevents the dopamine extracted from the striatal samples from breaking down.
For 100 ml of sample buffer:
= 100 ml HPLC grade H2O
= 0.862 ml PCA (70%) (Sigma)
= 61.5 mg glutathione (reduced)
Measure out the HPLC grade H2O, add the PCA, dissolve the glutathione in the solution, mix well and filter. The sample buffer can be stored at 4°C for up to 1 month. - Thaw striatal slices on ice, then homogenise in 200 μl sample buffer using an ultrasonic cell disrupter (Fisher Sonic, USA). Keep samples on ice at all times and homogenise each sample three times for 10 seconds each ensuring that the samples are allowed to cool down between homogenization periods.
- Set aside 20 μl of each homogenized sample (store at -80°C) for a protein assay.
- Centrifuge the samples (20,800 x g, 20 min (4°C, Eppendorf 58- 04R, USA)), then remove the supernatant and filter through a 0.2 μm PVDF membrane (Pall, Cat#: 1935-28143-310) and freeze at -80°C until HPLC analysis. Retain the protein pellet as a back-up for the protein assay.
- Preparation of the mobile phase for HPLC to run through the HPLC column.
For 1 L of mobile phase:
60 mM NaH2PO4.H2O (monobasic) = 8.27 g
30 mM Citric acid = 5.7 g
0.13 mM Ethylenediaminetetraacetic acid (EDTA) disodium salt dehydrate = 48 mg
0.16 mM Sodium dodecyl sulfate (SDS) = 45 mg
850 ml HPLC grade water (Caledon, Cat#: 8801-7)
15% v/v Acetonitrile-190 (pH 3.35) = 150 ml HPLC grade (Caledon, Cat#: 1401-7)
~2 μl of 10 M NaOH (in HPLC grade H2O)
All reagents should be ≥99.0% pure and of HPLC grade wherever possible. The water and acetonitrile must be HPLC grade. Mobile phase must be used within one week of preparation. Have dedicated, clean glassware and stir bars solely for the preparation of mobile phase (a 1 L glass measuring cylinder and at least 2 1 L glass flasks). Before preparing the mobile phase have two clean 1 L flasks prepared, one to make the solution in and one to transfer it into during filtration and degassing. Allow the mobile phase to circulate through the HPLC overnight and check that the baseline is flat prior to use. - Measure out 700 ml of HPLC grade water into a glass measuring cylinder and dissolve the NaH2PO4.H2O, citric acid, EDTA and SDS in it. Top up the water to 850 ml.
- Adjust the pH to 3.35 using 10 M NaOH, then add the acetonitrile and mix the solution using a Teflon coated stir bar.
- Put a stir bar in the second clean, empty 1 L flask and vacuum filter the mobile phase into it through a 0.22 μm HVLP Millipore filter: (Cat#: SLGP033RS). Wet the filter in 85:15 H2O/ACN before filtration. Once the mobile is fully filtered, degas the mobile phase by stirring it under vacuum for at least 10 min.
- Prepare the standards against which the dopamine concentration of the striatal samples will be measured. Standards are run through the HPLC at the beginning and end of each batch of striatal samples. A 1 mg/ml stock solution of dopamine can be made in advance and stored in 20 μl aliquots at -80°C. To prepare the stock solution dissolve 5 mg dopamine in 5 ml sample buffer (see above) and mix it thoroughly. Begin preparing the standards by taking 5 μl of 1 mg/ml stock solution, adding it to 2.5 ml sample buffer and mixing it thoroughly to obtain a 2 μg/ml dopamine solution.
- Four standards are run through the HPLC to produce a concentration curve. For dopamine the concentrations are: 100 ng/ml, 50 ng/ml, 25 ng/ml, and 12.5 ng/ml. In order to generate these four concentrations of dopamine take 10 μl of the 2 μg/ml dopamine solution prepared in 7.10). and add it to 190 μl of sample buffer to give you a 100 ng/ml dopamine solution. Following this perform a 1:1 serial dilution of the 100 ng/ml dopamine solution with sample buffer. Simply take 100 μl of 100 ng/ml dopamine and add 100 μl of sample buffer and mix to produce 50 ng/ml dopamine, then take 100 μl of 50 ng/ml dopamine and add 100 μl of sample buffer and mix to produce 25 ng/ml dopamine and so on.
- To prepare the HPLC system, (Waters 1525μ Binary pump, 717plus autosampler, Symmetry C-18 reverse-phase column (15 cm x 4.6 mm i.d., 5 μm particle size) (30°C), electrochemical detector (ESA Coulochem III) equipped with a dual electrode analytical cell (ESA 5011A) and controlled by Breeze software (Waters)) first purge the system with mobile phase by selecting the purge protocol in the computer software or by following the manufacturer's instructions. This exchanges all previous solution in the HPLC system with fresh mobile phase.
- Set the electrochemical potentials at 400 mV and -300 mV for the first and second electrodes respectively. Following this, equilibrate the system by running it for at least 2 hours and ideally overnight with the mobile phase at a flow rate of 1 ml/min. During equilibration the cells should be turned on and the baseline reading monitored, when the reading becomes stable the system is ready to be used. For the first hour of equilibration let the mobile phase that exits the system go into a waster container, following this you can allow the mobile phase to recirculate through the HPLC system by putting the outlet hose into the mobile phase flask.
- Thaw striatal samples on ice, then inject 20 μl of each dopamine standard followed by 2 x 20 μl of each sample and a further 20 μl of each dopamine standard into the equilibrated HPLC system with the mobile phase flow rate at 1 ml/min.
- Use the HPLC system software to analyse the area of the dopamine peaks produced by the standards and samples. Comparing the area of the dopamine peaks present in the striatal samples to the standard dopamine concentration curve will identify the amount of dopamine present in 20 μl of each striatal sample. Scale this figure up to find the total dopamine present in the whole striatal slice.
- Perform a standard protein assay on the 20 μl of striatal homogenate that you set aside in 7.4). This will measure the amount of protein present in the striatal samples in mg/ml. From this calculate the amount of dopamine in the striatal samples as a value in ng/mg allowing you to compare dopamine concentrations between hemispheres and between mice.
8. Representative Results
Fifteen to twenty one days following 6-OHDA-lesion surgery, when the amount of cell death caused by the neurotoxin has reached completion26, measurement of spontaneous 360° rotations following administration of saline (i.p.)28 can be used to assess the success of 6-OHDA-lesioned (Figure 2). This method of behavioural assessment is simple and quick, and avoids potential priming effects caused by dopaminergic agents such as amphetamine or apomorphine challenges. Furthermore, spontaneous rotation assessment is a better predictor of animals with <95% dopamine depletion compared to forelimb paw placement test27. By correlating striatal dopamine levels from striatal samples (prepared upon completion of behavioural studies using HPLC) with percentage of spontaneous ipsiversive rotations in each animal, we have found that animals which exhibit 70% or more ipsiversive rotations towards the 6-OHDA lesion have lost >95% striatal dopamine (Figure 3)27. Following partial 6-OHDA-induced lesions (59.44±17.20) (Figure 3) of the medial forebrain bundle, there was no rotational bias between ipsiversive versus contraversive sides when measuring spontaneous rotations (40.91±8.01) (Figure 2). Thus, as has been found following 6-OHDA administration to the MFB in rats, it is possible to cause a profound loss of dopaminergic nigro-striatal neurons, and saline administration (i.p.) is an accurate method for screening the extent of 6-OHDA lesion in these animals.
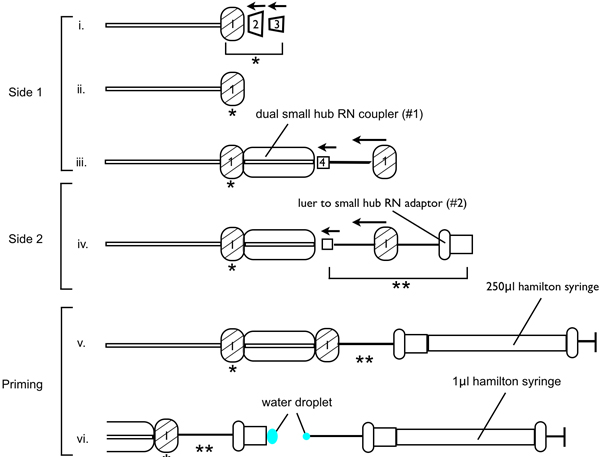
Figure 1. Schematic diagram to demonstrate assembly of the injection needle apparatus for 6-OHDA-lesion surgeries.
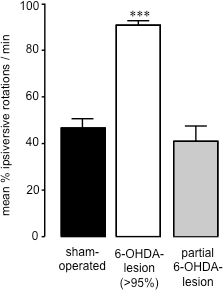
Figure 2. Assessment of rotational behaviour in sham-operated and 6-OHDA lesioned mice. Spontaneous rotations in sham-operated and 6-OHDA-lesioned mice. Data are presented as mean percentage ipsiversive rotations ± SEM, where net contraversive and ipsiversive rotations are 100%. Open bars: sham-operated animals; Grey bars: 6-OHDA-lesioned animals with >95% dopamine loss; Black bars: animals that underwent 6-OHDA-lesion surgery that were partially lesioned. *** P<0.001 compared to sham-operated animals. One-way ANOVA followed by Dunn's multiple comparison test (sham-operated: n=17; 6-OHDA-lesioned (>95%): n=23; partial 6-OHDA-lesion: n=3).
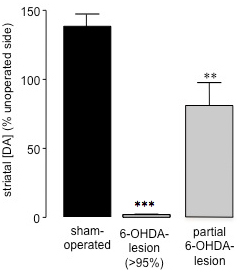
Figure 3. Assessment of striatal dopamine levels in sham-operated and 6-OHDA lesioned mice using HPLC. Striatal dopamine content was determined in the operated and unoperated hemisphere of sham and 6-OHDA-lesioned animals. Data are presented as mean percentage ± SEM of striatal dopamine levels in the unoperated striatum. Open bars: sham-operated animals; Grey bars: 6-OHDA-lesioned animals with >95% dopamine loss; Black bars: animals that underwent 6-OHDA-lesion surgery that were partially lesioned. *** P<0.001, ** P<0.01 compared to sham-operated animals. One-way ANOVA followed by Dunn's multiple comparison test (sham-operated: n=17, 6-OHDA-lesioned (>95%): n=23, partial 6-OHDA-lesion: n=3).
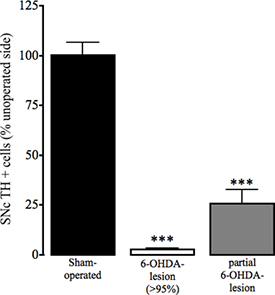
Figure 4. Assessment of tyrosine hydroxylase immunoreactivity in the SNc in sham-operated and 6-OHDA lesioned mice. As a marker of dopamine cell loss in the substantia nigra pars compacta (SNc), loss of tyrosine hydroxylase (TH) positive immunohistochemisty in the operated and unoperated hemisphere of sham and 6-OHDA-lesioned animals was determined as described in Thiele et al. In Press. Data are presented as mean percentage ± SEM of TH positive cells in the unoperated striatum. Open bars: sham-operated animals; Grey bars: 6-OHDA-lesioned animals with >95% dopamine loss; Black bars: animals that underwent 6-OHDA-lesion surgery that were partially lesioned. *** P<0.001, ** P<0.01 compared to sham-operated animals. One-way ANOVA followed by Dunn's multiple comparison test (sham-operated: n=17, 6-OHDA-lesioned (>95%): n=23, partial 6-OHDA-lesion: n=3).
Discussion
This protocol describes a method for the generation of a stable unilateral 6-OHDA-lesioned mouse model of Parkinson's disease, which is extremely reproducible, with a high lesion success rate, and a low mortality rate. The success of 6-OHDA lesion surgery can be easily estimated by the measurement of spontaneous rotational behaviour with >70% ipsiversive rotations indicative of >95% dopamine depletion in the lesioned striatum27. Quantification of striatal dopamine levels is the most accurate measurement ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Department of Foreign Affairs and International Trade (Government of Canada), University of Toronto Connaught Fund, the Canadian Foundation for Innovation, NSERC, the Krembil Foundation and the Cure Parkinson’s Trust.
Materials
| Name | Company | Catalog Number | Comments |
| Name of the reagent | Company | Catalogue number | Comments (optional) |
| desipramine HCl | Sigma-Aldrich, Oakville, ON, Canada | D125 | 25mg/kg |
| pargyline HCl | Sigma-Aldrich, Oakville, ON, Canada | P8013 | 5mg/kg |
| 6-OHDA HBr | Sigma-Aldrich, Oakville, ON, Canada | H116 | 3mg / mouse |
| stereotaxic Frame | Kopf Instruments, Tujunga, CA, USA | Model 900 | |
| mouse ear cups | Kopf Instruments, Tujunga, CA, USA | Model 921 Zygoma Ear Cups | |
| mouse incisor bar | Kopf Instruments, Tujunga, CA, USA | Model 923B | |
| mouse anaesthesia mask | Kopf Instruments, Tujunga, CA, USA | Model 923B | |
| priming kit (containing 250ml syringe) | Hamilton Company, Reno, NV, USA | PRMKIT 81120 | |
| RN compression fitting kit (1 mm) | Hamilton Company, Reno, NV, USA | 55750-01 | |
| PEEK tubing from RN compression fitting kit< (1/16th inch) | Hamilton Company, Reno, NV, USA | 55751-01 | |
| dual small hub RN Coupler | Hamilton Company, Reno, NV, USA | 55752-01 | |
| luer to small hub RN adaptor | Hamilton Company, Reno, NV, USA | 55753-01 | |
| 1ml 25S syringe model 7001KH | Hamilton Company, Reno, NV, USA | 80100 | |
| *33G removable needle (RN) pack of 6. . Custom 1 inch with 45<° bevel | Hamilton Company, Reno, NV, USA | 7803-05 | |
| Scissors | Fine Science Tools, Vancouver, BC, Canada. | 14084-08 | |
| Scalpel | Fine Science Tools, Vancouver, BC, Canada | 10003-12 | |
| Scalpel blades | Fine Science Tools, Vancouver, BC, Canada | 10035-20 | |
| Forcep | Fine Science Tools, Vancouver, BC, Canada | 11608-15 | |
| Hemostats | Fine Science Tools, Vancouver, BC, Canada. | 13004-14 | |
| Isoflurane | Abbot | 02241315 | 2-3% |
| Suters (Vicryl 4.0) | Syneture | SS-683 | |
| Steriliser | Fine Science Tools, Vancouver, BC, Canada | 18000-45 | |
| Infusion Pump | Harvard Apparatus | PhD 22/2000 | |
| Needles (27G) | Becton Dickinson | 305109 | |
| Needles (25G) | Becton Dickinson | 305127 | |
| Syringes (1ml) | BD syringe | 309692 | |
| Anaesthesia trolley | LEI medical | M2000 | |
| Baytril | CDMV, St. hyacinthe, QC | 102207 | |
| Lidocaine | CDMV, St. hyacinthe, QC | 3914 | |
| Betadine solution | CDMV, St. hyacinthe, QC | 19955 |
References
- Costall, B., Naylor, R. J., Pycock, C. Non-specific supersensitivity of striatal dopamine receptors after 6-hydroxydopamine lesion of the nigrostriatal pathway. Eur. J. Pharmacol. 35, 276-283 (1976).
- Maneuf, Y. P., Mitchell, I. J., Crossman, A. R., Brotchie, J. M. On the role of enkephalin cotransmission in the GABAergic striatal efferents to the globus pallidus. Exp. Neurol. 125, 65-71 (1994).
- Robertson, G. S., Robertson, H. A. Evidence that L-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J. Neurosci. 9, 3326-3331 (1989).
- Ungerstedt, U., Arbuthnott, G. W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 24, 485-493 (1970).
- Brotchie, J. M. Novel approaches to the symptomatic treatment of parkinsonian syndromes: alternatives and adjuncts to dopamine-replacement. Curr. Opin. Neurol. 10, 340-345 (1997).
- Besson, M. J., Graybiel, A. M., Nastuk, M. A. [3H]SCH 23390 binding to D1 dopamine receptors in the basal ganglia of the cat and primate: delineation of striosomal compartments and pallidal and nigral subdivisions. Neuroscience. 26, 101-119 (1988).
- Gerfen, C. R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 250, 1429-1432 (1990).
- Schiffmann, S. N., Jacobs, O., Vanderhaeghen, J. J. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J. Neurochem. 57, 1062-1067 (1991).
- Hutchison, W. D. Differential neuronal activity in segments of globus pallidus in Parkinson's disease patients. Neuroreport. 5, 1533-1537 (1994).
- Pan, H. S., Penney, J. B., Young, A. B. Gamma-aminobutyric acid and benzodiazepine receptor changes induced by unilateral 6-hydroxydopamine lesions of the medial forebrain bundle. J. Neurochem. 45, 1396-1404 (1985).
- Pan, H. S., Walters, J. R. Unilateral lesion of the nigrostriatal pathway decreases the firing rate and alters the firing pattern of globus pallidus neurons in the rat. Synapse. 2, 650-656 (1988).
- Gong, S. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 425, 917-925 (2003).
- Kreitzer, A. C., Malenka, R. C. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 445, 643-647 (2007).
- Shen, W., Flajolet, M., Greengard, P., Surmeier, D. J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 321, 848-851 (2008).
- Lundblad, M., Picconi, B., Lindgren, H., Cenci, M. A. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 16, 110-123 (2004).
- Grealish, S., Mattsson, B., Draxler, P., Bjorklund, A. Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson's disease. Eur. J. Neurosci. 31, 2266-2278 (2010).
- Dauer, W., Przedborski, S. Parkinson's disease: mechanisms and models. Neuron. 39, 889-909 (2003).
- Francardo, V. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson's disease. Neurobiol. Dis. 42, 327-340 (2011).
- Jakowec, M. W., Petzinger, G. M. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned model of parkinson's disease, with emphasis on mice and nonhuman primates. Comp. Med. 54, 497-513 (2004).
- Visanji, N. P., Brotchie, J. M. MPTP-Induced Models of Parkinson's Disease in Mice and Non-Human Primates. Curr. Protoc. Pharmacol. Chapter 5, 42-42 (2005).
- Sedelis, M., Schwarting, R. K., Huston, J. P. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav. Brain Res. 125, 109-125 (2001).
- Schallert, T., Fleming, S. M., Leasure, J. L., Tillerson, J. L., Bland, S. T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 39, 777-787 (2000).
- Paul, M. L., Currie, R. W., Robertson, H. A. Priming of a D1 dopamine receptor behavioural response is dissociated from striatal immediate-early gene activity. Neuroscience. 66, 347-359 (1995).
- Breese, G. R., Traylor, T. D. Effect of 6-hydroxydopamine on brain norepinephrine and dopamine evidence for selective degeneration of catecholamine neurons. J. Pharmacol Exp. Ther. 174, 413-420 (1970).
- Breese, G. R., Chase, T. N., Kopin, I. J. Metabolism of tyramine-3H and octopamine-3H by rat brain. Biochem. Pharmacol. 18, 863-869 (1969).
- Frankin, K. B. J., Paxinos, G. . The Mouse Brain in Stereotaxic Coordinates. , (2008).
- Cornell-Bell, A. H., Finkbeiner, S. M., Cooper, M. S., Smith, S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 247, 470-473 (1990).
- Iancu, R., Mohapel, P., Brundin, P., Paul, G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav. Brain Res. 162, 1-10 (2005).
- Tan, Y., Williams, E. A., Lancia, A. J., Zahm, D. S. On the altered expression of tyrosine hydroxylase and calbindin-D 28kD immunoreactivities and viability of neurons in the ventral tegmental area of Tsai following injections of 6-hydroxydopamine in the medial forebrain bundle in the rat. Brain Res. 869, 56-68 (2000).
- Thiele, S. L. Generation of a model of L-DOPA-induced dyskinesia in two different mouse strains. J. Neurosci. Methods. 197, 193-208 (2011).
- Henry, B., Crossman, A. R., Brotchie, J. M. Characterization of a rodent model in which to investigate the molecular and cellular mechanisms underlying the pathophysiology of L-dopa-induced dyskinesia. Adv. Neurol. 78, 53-61 (1998).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved