A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Saliva, Salivary Gland, and Hemolymph Collection from Ixodes scapularis Ticks
In This Article
Summary
The collection of infected tick hemolymph, salivary glands, and saliva is important to study how tick-borne pathogens cause disease. In this protocol we demonstrate how to collect hemolymph and salivary glands from feeding Ixodes scapularis nymphs. We also demonstrate saliva collection from female I. scapularis adults.
Abstract
Ticks are found worldwide and afflict humans with many tick-borne illnesses. Ticks are vectors for pathogens that cause Lyme disease and tick-borne relapsing fever (Borrelia spp.), Rocky Mountain Spotted fever (Rickettsia rickettsii), ehrlichiosis (Ehrlichia chaffeensis and E. equi), anaplasmosis (Anaplasma phagocytophilum), encephalitis (tick-borne encephalitis virus), babesiosis (Babesia spp.), Colorado tick fever (Coltivirus), and tularemia (Francisella tularensis) 1-8. To be properly transmitted into the host these infectious agents differentially regulate gene expression, interact with tick proteins, and migrate through the tick 3,9-13. For example, the Lyme disease agent, Borrelia burgdorferi, adapts through differential gene expression to the feast and famine stages of the tick's enzootic cycle 14,15. Furthermore, as an Ixodes tick consumes a bloodmeal Borrelia replicate and migrate from the midgut into the hemocoel, where they travel to the salivary glands and are transmitted into the host with the expelled saliva 9,16-19.
As a tick feeds the host typically responds with a strong hemostatic and innate immune response 11,13,20-22. Despite these host responses, I. scapularis can feed for several days because tick saliva contains proteins that are immunomodulatory, lytic agents, anticoagulants, and fibrinolysins to aid the tick feeding 3,11,20,21,23. The immunomodulatory activities possessed by tick saliva or salivary gland extract (SGE) facilitate transmission, proliferation, and dissemination of numerous tick-borne pathogens 3,20,24-27. To further understand how tick-borne infectious agents cause disease it is essential to dissect actively feeding ticks and collect tick saliva. This video protocol demonstrates dissection techniques for the collection of hemolymph and the removal of salivary glands from actively feeding I. scapularis nymphs after 48 and 72 hours post mouse placement. We also demonstrate saliva collection from an adult female I. scapularis tick.
Protocol
1. Hemolymph collection for slide preparation
(Movie 1)
- Gently remove actively feeding ticks from an animal and place in 3% topical hydrogen peroxide for 5 minutes and then in 70% ethanol for 10 minutes to surface sterilize.
- With a pap pen draw a circle onto a silane coated microscope slide and place the tick within the pap pen circle.
- Silane coated slides are used for best adherence of the hemolymph to the microscope slide.
- View the tick under a dissecting microscope (1X objective, 10X eyepiece, 3.5X magnification).
- Gently push down on the tick's dorsum with forceps to splay the tick's legs and immobilize the tick.
Note: When immobilizing the tick do not press too hard because this may disrupt the midgut or puncture the tick and contaminate the hemolymph.
- Amputate the tick's leg or legs at the distal joint with a fine point disposable scalpel. To determine infectivity via hemolymph only 1 leg needs to be amputated. For collection of hemolymph on a slide several legs may be amputated.
Note: Do not cut the leg too close to the body because this may cause midgut contamination of the hemolymph.
- After the leg or legs are cut continue to gently apply pressure to the tick's dorsum for the hemolymph to secrete out of the legs onto the slide. Gently move the tick around on the slide to spread the hemolymph.
2. Salivary gland removal
(Movies 2 & 3)
- Spot several 25 μl pools of phosphate buffered saline (PBS) onto a microscope slide and place a tick into one of the PBS pools.
- View the tick under a dissecting microscope (1X objective, 10X eyepiece, 3.5X magnification).
- Stabilize the tick with fine tipped forceps by holding the basis capitulum (mouth parts) or the rear of the tick.
- Insert the fine tipped forceps into the rear of the tick and slice up the tick's dorsum to expose the organs. If desired the midgut can be removed at this time and transferred to a fresh pool of PBS on a microscope slide or to a microfuge tube containing PBS.
- Find the pair of salivary glands (grape-like clusters) located bilaterally alongside the legs of the tick. If the salivary glands are not visible amongst the tick debris move the main tick portion, still containing the salivary glands, to a fresh pool of PBS to reduce the disruption and loss of the salivary glands.
Note: Earlier in a feeding, the salivary glands are harder to locate because they are not as developed as compared to later on in the feeding.
- Remove the salivary glands from the tick with fine tipped forceps and place in a fresh pool of PBS.
- With fine tipped forceps transfer the salivary glands to another clean 25 μl PBS pool, repeat this wash step 3-4 more times to remove any external microorganisms and tick debris.
Note: Wash the salivary gland clusters gently to reduce the loss and disruption of individual salivary glands.
- Place the glands into a clean pool of PBS on a silane coated slide or in a microfuge tube containing PBS.
3. Saliva collection
(Movie 4)
- Gently remove adult female ticks from the rabbit or other host using fine tipped forceps just before they drop off fully engorged, approximately 5-7 days post-attachment.
- Adhere the nearly engorged tick onto one end of a microscope slide with scotch tape. The tape should be placed approximately ¾ of the way up the tick's dorsum toward the head, leaving the tick's basis capitulum (mouth parts) exposed.
- Where the tape meets the anterior edge of the ticks dorsal surface pipet 5 μl of 5% pilocarpine solution (in methanol). Allow the tape to wick the pilocarpine over the tick's dorsum, without allowing the pilocarpine to come in contact with the tick's basis capitulum.
- Mount a piece of non-toxic modeling clay onto the microscope slide approximately one inch from the tick's mouthparts.
- Using fine tipped forceps break off the tip of a pulled capillary tube28 to the desired diameter.
- View the tick's basis capitulum under a dissecting microscope.
- Gently fit the tick's hypostome into the pulled capillary tube allowing the maxillary palps to reside on the outside of the capillary tube.
- Press the opposite end of the capillary tube into the modeling clay to hold the capillary tube in place.
- Place the mounted salivating tick inside a dark chamber with high humidity (e.g., a lidded styrofoam box lined with wet paper towels). Tilt the slide so the hypostome points to the bottom of the container, allowing gravity to aid in saliva collection.
- Place the container at room temperature.
- Closely monitor the salivating ticks for the first hour, and collect saliva as it is generated by expelling it out of the capillary tubes with a Pasteur pipet bulb. After the first hour, check accumulation every hour for at least 4 hours. Continue to collect the saliva as it is generated.
Note: Saliva acquisition can be stopped once enough saliva is collected for the study being performed.
- If the ticks are not salivating or if more saliva is required, salivation can occasionally be induced by using the capillary tube to massage the hypostome.
- Add 0.1 volumes of protease inhibitor cocktail to the saliva and store at -80°C until needed.
4. Representative Results
Movie 1 demonstrates how to hold a partially fed I. scapularis nymph and amputate the legs to collect hemolymph onto a microscope slide. Once the leg or legs are amputated a clear fluid is secreted (figure 1A and 1B). If the midgut is ruptured the hemolymph appears cloudy as it is comes out of the amputated leg(s) (figure 1C and 1D).
The extraction of salivary glands after the nymph has been feeding for 48 or 72 hours is demonstrated in movies 2 and 3. After the tick is punctured there is generally a lot of debris (consisting of trachea, malpighian tubules, blood, connective tissue etc.), to prevent the loss or disruption of the salivary glands move the tick to a fresh pool of PBS. Figures 2A and 2B show where the salivary glands are located after the nymph has been cut open and figure 2C shows removed salivary gland clusters in a pool of PBS.
Saliva collection set up from I. scapularis adult females is shown in movie 4 and figure 3. A tick salivating into a capillary tube is observed in movie 5. This method of saliva collection used pilocarpine to stimulate salivation and can yield over 20 μl of saliva per adult female tick.
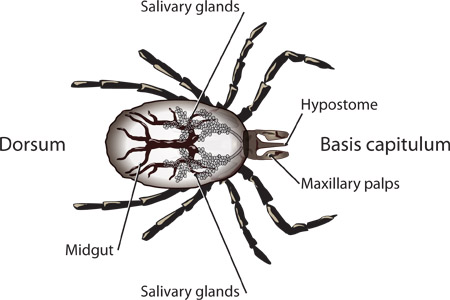
Figure 1. Labeled structures of an I. scapularis nymph.
Movie 1. Ixodes scapularis hemolymph collection. Click here to watch movie.
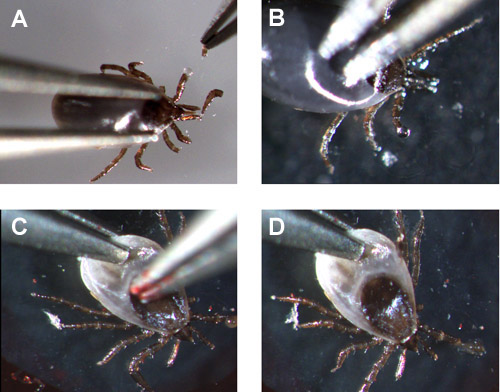
Figure 2. Uncontaminated (A & B) and contaminated (C & D) hemolymph exuding from the nymph's leg.
Movie 2. Salivary gland extraction from a 48 hour fed I. scapularis nymph.Click here to watch movie.
Movie 3. Salivary gland extraction from a 72 hour fed I. scapularis nymph. Click here to watch movie.
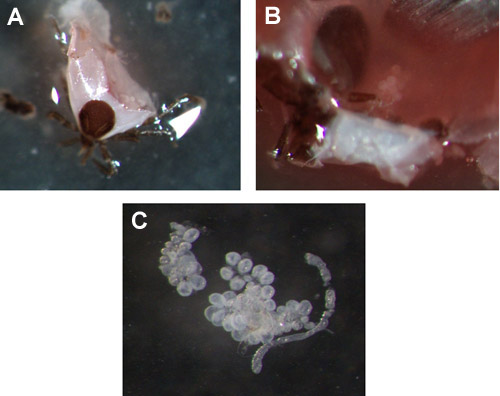
Figure 3. Ixodes scapularis nymph salivary glands. (A & B) Examples of salivary glands in a 72 hour fed nymph, prior to extraction. (C) Removed salivary gland cluster.
Movie 4. Saliva collection set up from an adult I. scapularis female tick. Click here to watch movie.
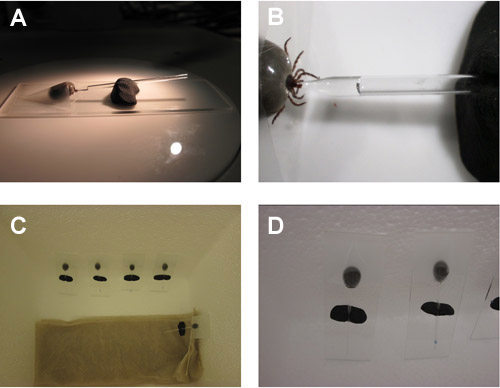
Figure 4. Saliva collection from adult I. scapularis female ticks. (A & B) Tick mounted on a slide with its hypostome in the pulled end of the capillary tube with the unpulled end of the capillary tube held by modeling clay. (C & D) Humidified chamber containing salivating adult I. scapularis female ticks.
Movie 5. Ixodes scapularis female tick salivating into a capillary tube. Click here to watch movie.
Discussion
The collection of tick hemolymph, salivary glands, and saliva is important in the study of tick-borne pathogen transmission, prevalence, dissemination, proliferation, and persistence in both the tick and the host 6,11-13,20,23,29. There are several ways to dissect a tick30,31. However, when collecting salivary glands it is critical to dissect the tick properly so the salivary glands are not ruptured or lost in the tick's remains. Once the salivary glands are removed from the tick they need to be was...
Disclosures
We have nothing to disclose.
Acknowledgements
The authors would like to thank the Division of Vector-Borne Diseases Animal Resources Branch, specifically Andrea Peterson, Lisa Massoudi, Verna O'Brien, and John Liddell for their care and maintenance of the mice and rabbits. We would also like to thank Amy Ullmann, Theresa Russell, and Barbara J. Johnson for their contributions toward this manuscript. Finally, we would like to acknowledge Alissa Eckert in the Office of the Associate Director for Communication at the CDC for producing the graphic illustrations and Judy Lavelle for directing all the legalities associated with the filming of this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| Reagent | Company | Catalogue Number | |
| Hydrogen peroxide | Fisher | H312-500 | |
| Ethanol | Acros | 61509-5000 | |
| PBS | Boston Bioproducts | BM-2205 | |
| Dumont Fine forceps (3C) | Fisher | NC9906085 | |
| Silane treated microscope slides | Bioworld | 42763007-1 | |
| Pap pen | Bioworld | 21750008-1 | |
| Super frost plus microscope slides | Fisher | 12-550-18 | |
| Pilocarpine | Sigma | P6503-5G | |
| Protease inhibitor cocktail | Sigma | P2714 | |
| #11 disposable scalpel | Feather | 2975#11 | |
| Nontoxic modeling clay | Fisher | S17307 | |
| Capillary tubes | Chase scientific Glass, inc | 40A502 |
References
- Quach, K. A., Boctor, F. N., Elston, D. M. What's eating you? Hyalomma ticks. Cutis. 87, 165-167 (2011).
- Graham, J., Stockley, K., Goldman, R. D. Tick-borne illnesses: a CME update. Pediatr. Emerg. Care. 27, 141-147 (2011).
- Nuttall, P. A., Paesen, G. C., Lawrie, C. H., Wang, H. Vector-host interactions in disease transmission. J. Mol. Microbiol. Biotechnol. 2, 381-386 (2000).
- Estrada-Pena, A., Jongejan, F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 23, 685-715 (1999).
- Nuttall, P. A. Pathogen-tick-host interactions: Borrelia burgdorferi and TBE virus. Zentralbl Bakteriol. 289, 492-505 (1999).
- Jones, L. D., Hodgson, E., Nuttall, P. A. Enhancement of virus transmission by tick salivary glands. J. Gen. Virol. 70, 1895-1898 (1989).
- Labuda, M., Nuttall, P. A. Tick-borne viruses. Parasitol. 129, 221-245 (2004).
- Socolovschi, C., Mediannikov, O., Raoult, D., Parola, P. Update on tick-borne bacterial diseases in Europe. Parasite. 16, 259-273 (2009).
- Zhang, L., et al. Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph. PLoS Pathog. 7, e1002079 (2011).
- Piesman, J., Schneider, B. S. Dynamic changes in Lyme disease spirochetes during transmission by nymphal ticks. Exp. Appl. Acarol. 28, 141-145 (2002).
- Brossard, M., Wikel, S. K. Tick immunobiology. Parasitol. , S161-S176 (2004).
- Machackova, M., Obornik, M., Kopecky, J. Effect of salivary gland extract from Ixodes ricinus ticks on the proliferation of Borrelia burgdorferi sensu stricto in vivo. Folia Parasitol. 53, 153-158 (2006).
- Nuttall, P. A., Labuda, M. Tick-host interactions: saliva-activated transmission. Parasitol. 129, 177-189 (2004).
- Anguita, J., Hedrick, M. N., Fikrig, E. Adaptation of Borrelia burgdorferi in the tick and the mammalian host. FEMS Microbiol. Rev. 27, 493-504 (2003).
- Hovius, J. W., van Dam, A. P., Fikrig, E. Tick-host-pathogen interactions in Lyme borreliosis. Trends Parasitol. 23, 434-438 (2007).
- Dunham-Ems, S. M., et al. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. Journal Clin. Invest. 119, 3652-3665 (2009).
- Ribeiro, J. M., Mather, T. N., Piesman, J., Spielman, A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J. Med. Entomol. 24, 201-205 (1987).
- Piesman, J. Transmission of Lyme disease spirochetes (Borrelia burgdorferi. Exp. Appl. Acarol. 7, 71-80 (1989).
- De Silva, A. M., Fikrig, E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 53, 397-404 (1995).
- Horka, H., Cerna-Kyckova, K., Skallova, A., Kopecky, J. Tick saliva affects both proliferation and distribution of Borrelia burgdorferi spirochetes in mouse organs and increases transmission of spirochetes to ticks. Int. J. Med. Microbiol. 299, 373-380 (2009).
- Brossard, M., Wikel, S. K. Immunology of interactions between ticks and hosts. Med. Vet. Entomol. 11, 270-276 (1997).
- Wikel, S. K. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29 (99), 851-859 (1999).
- Binnington, K. C., Kemp, D. H. Role of tick salivary glands in feeding and disease transmission. Adv. Parasitol. 18, 315-339 (1980).
- Guo, X., et al. Inhibition of neutrophil function by two tick salivary proteins. Infect. Immun. 77, 2320-2329 (2009).
- Montgomery, R. R., Lusitani, D., De Boisfleury Chevance, A., Malawista, S. E. Tick saliva reduces adherence and area of human neutrophils. Infect. Immun. 72, 2989-2994 (2004).
- Lima, C. M., et al. Differential infectivity of the Lyme disease spirochete Borrelia burgdorferi derived from Ixodes scapularis salivary glands and midgut. J. Med. Entomol. 42, 506-510 (2005).
- Severinova, J., et al. Co-inoculation of Borrelia afzelii with tick salivary gland extract influences distribution of immunocompetent cells in the skin and lymph nodes of mice. Folia Microbiol. 50, 457-463 (2005).
- Labuda, M., Jones, L. D., Williams, T., Nuttall, P. A. Enhancement of tick-borne encephalitis virus transmission by tick salivary gland extracts. Med. Vet. Entomol. 7, 193-196 (1993).
- Kariu, T., Coleman, A. S., Anderson, J. F., Pal, U. Methods for Rapid Transfer and Localization of Lyme Disease Pathogens Within the Tick Gut. J. Vis. Exp. (48), e2544 (2011).
- Edwards, K. T., Goddard, J., Varela-Stokes, A. S. Examination of the internal morphology of the Ixodid tick Amblyomma maculatum koch, (Acari:Ixodidae); a "How-to" pictorial dissection guide. Midsouth Entomologist. 2, 28-39 (2009).
- Ledin, K. E., et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med. Vet. Entomol. 19, 90-95 (2005).
- Ribeiro, J. M., Zeidner, N. S., Ledin, K., Dolan, M. C., Mather, T. N. How much pilocarpine contaminates pilocarpine-induced tick saliva?. Med. Vet. Entomol. 18, 20-24 (2004).
- Barker, R. W., Burris, E., Sauer, J. R., Hair, J. A. Composition of tick oral secretions obtained by three different collection methods. J. Med. Entomol. 10, 198-201 (1973).
- Burgdorfer, W. Hemolymph test. A technique for detection of rickettsiae in ticks. Am. J. Trop. Med. Hyg. 19, 1010-1014 (1970).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved