A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Real-time Digital Imaging of Leukocyte-endothelial Interaction in Ischemia-reperfusion Injury (IRI) of the Rat Cremaster Muscle
In This Article
Summary
Digital intravital epifluorescence microscopy of postcapillary venules in the cremasteric microcirculation is a convenient method to gain insights into leukocyte-endothelial interaction in vivo in ischemia-reperfusion injury (IRI) of striated muscle tissue. We here provide a detailed protocol to safely perform the technique and discuss its applications and limitations.
Abstract
Ischemia-reperfusion injury (IRI) has been implicated in a large array of pathological conditions such as cerebral stroke, myocardial infarction, intestinal ischemia as well as following transplant and cardiovascular surgery.1 Reperfusion of previously ischemic tissue, while essential for the prevention of irreversible tissue injury, elicits excessive inflammation of the affected tissue. Adjacent to the production of reactive oxygen species, activation of the complement system and increased microvascular permeability, the activation of leukocytes is one of the principle actors in the pathological cascade of inflammatory tissue damage during reperfusion.2, 3 Leukocyte activation is a multistep process consisting of rolling, firm adhesion and transmigration and is mediated by a complex interaction between adhesion molecules in response to chemoattractants such as complement factors, chemokines, or platelet-activating factor.4
While leukocyte rolling in postcapillary venules is predominantly mediated by the interaction of selectins5 with their counter ligands, firm adhesion of leukocytes to the endothelium is selectin-controlled via binding to intercellular adhesion molecules (ICAM) and vascular cellular adhesion molecules (VCAM).6, 7
Gold standard for the in vivo observation of leukocyte-endothelial interaction is the technique of intravital microscopy, first described in 1968.8
Though various models of IRI (ischemia-reperfusion injury) have been described for various organs, 9-12 only few are suitable for direct visualization of leukocyte recruitment in the microvascular bed on a high level of image quality.8
We here promote the digital intravital epifluorescence microscopy of the postcapillary venule in the cremasteric microcirculation of the rat 13 as a convenient method to qualitatively and quantitatively analyze leukocyte recruitment for IRI-research in striated muscle tissue and provide a detailed manual for accomplishing the technique. We further illustrate common pitfalls and provide useful tips which should enable the reader to truly appreciate, and safely perform the method.
In a step by step protocol we depict how to get started with respiration controlled anesthesia under sufficient monitoring to keep the animal firmly anesthetized for longer periods of time. We then describe the cremasteric preparation as a thin flat sheet for outstanding optical resolution and provide a protocol for leukocyte imaging in IRI that has been well established in our laboratories.
Protocol
1. Anesthesia and Monitoring
- Appropriate national and institutional ethics should be in place before performing animal experiments. Following approval from the ethics committee anesthetize male Sprague Dawley rats with a body weight from 120 - 180 g. Deliver 2 - 3 vol% isoflurane to a Plexiglas box via isoflurane vaporizer and place the rat inside.
- As soon as the appropriate level of anesthesia is achieved (lack of reaction to toe or tail pinch) the rat is weighted and shaved on the ventral cervical area.
- Place the rat in dorsal recumbency on a heating pad to maintain body temperature at 37 °C and apply isoflurane at 2 vol% using a silicone mask.
The following preparation steps are best accomplished using a surgical microscope.
- For the preparation of the trachea, the carotid artery and the jugular vein, perform a 2 cm horizontal skin incision in the area of the suprasternal notch and mobilize the salivary glands laterally.
- You now face the ventral neck muscles. Carefully separate them in the midline and find the trachea. Expose 1 - 2 cm of the trachea and place a micro forceps under it so as to raise it up.
- Now slit about halfway through the ventral side of the trachea. Be careful not to cut all the way through, if you do, the cut end of the trachea will slip back into the chest and be very difficult to work with.
- Insert an Abbocath tube (14G) used as tracheal tube into the lower part of the trachea, which has previously been connected to an animal ventilator. Tip: Firm fixation on magnet clamps as well as suturing of the trachea around the tube is essential to keep the tracheal tube in place. Generally we use Terylene 5/0 suture, however similar suture material can be used.
- Respiration can then be volume controlled (frequency, 35 - 45 breaths/minute; tidal volume, 4.5 - 5 ml; FiO2, 0.35 - 0.50; Isoflurane 1.5 - 2 vol%).14 Atelectasis can be prevented by maintaining a positive end-expiratory pressure of 5 to 10 mm of H2O.15
- For the carotid canulation, the right sternohyoid muscle is separated by blunt dissection to locate the carotid artery.
- Separate the vagus nerve carefully from the carotid artery and mount the artery on an angled micro forceps to stop blood flowing from the heart.
- Pass two pieces of equal length of suture beneath the carotid artery. In reference to the heart, the more distal suture is tied tightly to occlude blood flowing from the head region whereas the proximal suture is tied loosely around the carotid artery.
- Using micro scissors a small cut is made into the carotid artery between the two ligatures.
- Insert a polyethylene catheter (0.28 mm inner diameter) filled with normal saline solution that is connected to a pressure transducer.
- Remove the micro forceps and thread the catheter further into the artery. Then tighten the proximal ligature around the artery and catheter. Tip: Application of a second proximal suture prevents bleeding after removing the micro forceps.
- Tie the distal ligature around the carotid and the catheter for additional anchorage.
- Monitor anesthesia continuously by monitoring heart rate and arterial pressure. Perform intermittent arterial blood gas analyses using a blood gas analyzer.16 A heart rate lower than 300 bpm or exceeding 360 bpm as well as a mean arterial pressure dropping below 80 mmHg for longer than 5 minutes are criteria for exclusion.17, 18 Maintain blood pH within physiological limits (7.35 - 7.45). In case the experiment has to be terminated due to abnormal monitoring rates, scarify the animal by neck dislocation or exanguation.
For the intravenous application of fluorescent dyes or other drugs of interest, perform the following:
- Focus the area of the left jugular vein with the surgical microscope.
- With forceps in each hand, tear the thin fascia to reveal the jugular vein. Mount the vein on an angled micro forceps. Blood flow will then stop.
- Using forceps, two pieces of equal length of suture are passed under the jugular vein concordant to the carotid canulation. Tie the distal suture tightly and the proximal suture loosely around the jugular vein.
- Make a small cut into the jugular vein and insert a polyethylene catheter (0.28 mm inner diameter) that has been flushed with saline. Mark that incision and canulation can be more demanding and time consuming than that of the carotid.
- Thread the catheter toward the heart and tighten the ligature closest to the heart around the vein and the catheter.
- After conforming patency, tie distal ligature around the catheter. Tip: Additional fixation of both catheters with tape may prevent dislodgment.
- Rinse catheters frequently to prevent intraluminal clotting.
2. Preparation of the Cremaster Muscle
- We use an aluminium stage of 1.5 - 2 cm thickness for cremasteric imaging. The stage rapidly adopts the desired temperature of the heating pad thus alleviating thermal control of the cremasteric tissue. This is crucial for inflammation studies. Alternatively a Plexiglas platform may be used though regulation of cremasteric temperature is more difficult. Place the scrotum in the centre of the stage.
- The initial incision is made in the skin and external spermatic fascia above the scrotum in the very distal end followed by cautious dilatation of the subdermal space using fine scissors. Avoid touching the underlying tissue with the instruments.
- As soon as the tissue is exposed it is moistened with pre-heated (37 °C) phosphate buffered saline solution with calcium and magnesium. Apply the solution regularly to any exposed tissue.
- Connective tissue between the external spermatic fascia and the cremaster muscle is carefully removed to free the cremaster muscle from surrounding tissue.
- The exterior surface of the cremaster muscle is then cautiously cleared of connective tissue. Continuous superfusion during dissection hydrates the connective tissue, facilitating visibility and removal.
- Suture the distal end of the cremaster sack to hold down and slightly extend the end of the sack.
- Incise the distal end of the sack on the ventral aspect and elongate the incision proximally using micro scissors. Carefully cauterize bleeding vessels along the incision lines utilizing a thermal cautery. Avoid unnecessary cauterization to limit additional inflammatory stimuli. Tip: Blood flow dynamics near the periphery of the preparation are altered by this tissue damage.19 Therefore, to minimize these affects on data collection, blood vessels near the center of the preparation should be used for imaging.
- The open cremaster lies flat on the aluminum pedestal though still connected by a thin ligament to the epididymis underneath the testicle. Reflecting the testicle to one side exposes this ligament including a small artery and vein which connect to the epididymis. Use the cautery to seal the vessels and employ the micro scissors to cut the connective ligament between testicle and cremasteric tissue.
- Gently push back the isolated testicle into the inguinal canal. Other authors resect the testicle after proximal ligature (orchiectomy) along with the associated inguinal fad pad.20 In a sensitive model as IRI induced leukocyte activation we try to avoid all additional surgical stimuli, therefore we refrain from resecting the testis, which is usually not necessary to get sufficient exposure of the cremaster muscle.
- In addition to the first fixation suture four edges of tissue (two on each side) and attach the threads to tapes to cautiously spread the cremasteric tissue radially on the aluminum stage. Leaving a gap between the aluminum stage and the external entrance of the inguinal canal simplifies subsequent cremasteric clipping for ischemia.
- Place two ends of polyethylene catheter (0.28 mm inner diameter) close to the cremasteric tissue for the superfusion setup. Make sure that the lumen is free of air to avoid air bubbles in the eventual fluid chamber.
- Draw a line of petroleum jelly (Vaseline) around the cremaster muscle using a syringe. The line must extend the size of the cover slip that is used for coverage.
- To create a fluid chamber, place a square cover slip (32×32 mm) over the cremasteric tissue and firmly attach the edges to the Vaseline line.
- In case the cremasteric tissue is not superfused with a specific drug, continuous replacement of the surrounding fluid is nonessential. A single application of phosphate buffered saline solution with calcium and magnesium into the created chamber may then be sufficient. Local stimulation with drugs however should be performed continuously via micro-perfusion pump at a rate of 3 ml per hour.
- The cremasteric tissue is now ready for microscopic imaging.
3. Intravital Setup
The basic intravital setup may vary. For epifluorescence imaging the experiments should be executed in a dark room.
- The animal is transferred to the stage of an intravital epifluorescence microscope equipped with a 470 nm LED luminous source for epi-illumination. Use a water immersion objective (20×/1.0) to achieve magnification of approximately 800×. Record observations by means of a high resolution digital camera and store records on a personal computer for offline evaluation.
- For leukocyte labeling, inject rhodamine 6G intravenously via the jugular catheter at concentrations of 0.4 mg/kg body weight.
- Choose a postcapillary venule for observation. Vessel size should range between 20-60 μm and blood flow should be sufficient. To minimize the influence of pre-activation of the tissue, only vessels in which leukocyte rolling is <20 cells/30 seconds and the number of adherent cells <10 cells/200μm of venular endothelium may be utilized for further analysis.
- If possible, up to three postcapillary venules can be used for observation; however they should be located in a corresponding section of the cremasteric tissue to avoid confusing the vessels at different time points.
4. Ischemia-reperfusion Injury (IIR)
- Let tissue stabilize for 30 minutes.
- Perform a recording of 30 seconds to establish basal values for leukocyte rolling and adherence. Ideally, generate in total three basal recordings to verify cell numbers and to obtain standard deviations.
- Gently place a Biemer vessel clip around the very proximal end of the exposed cremasteric tissue by using the application forceps. Stasis should occur immediately and can be visualized by epifluorescence microscopy in the observed vessel section.
- A variety of time courses can be utilized depending on the level of damage required. We apply 30 minutes of ischemia time as described by other authors.21-23 Remove the vessel clamp afterwards. Allow blood stream to stabilize for another 15 minutes.
- Subsequent recordings of 30 seconds duration can be made throughout the reperfusion period (e.g. 30 seconds every 15 minutes). Save recordings digitally for offline analysis.
- For the termination of the experiment, the rat is euthanized by cervical dislocation under sufficient anesthesia by pressing a blunt instrument such as the dull edge of a scissor blade at the base of the skull. With the other hand, the base of the tail is quickly pulled, causing separation of the cervical vertebrae from the skull.
5. Offline Video Playback Analysis
- For offline video playback analysis it is helpful to use software that allows selection of distinct pictures and observation of the video sequence in slow motion. Adjust contrast and brightness appropriately. We use software provided by the manufacturer of the microscope that allows length measurements and digital magnification.
- For the quantification of leukocyte rolling, define a virtual line that intersects the vessel vertically that is consistent in all records. Count the number of rolling leukocytes that pass the line within 30 seconds manually.
- For the quantification of adherent leukocytes, define a 200 μm vessel section that is consistent in all records.23 Count the number of clearly visible leukocytes that remain static during 30 seconds - thus defined as adherent.24
6. Representative Results
IRI of the cremaster muscle has no effect on mean arterial pressure (MAP), heart rate and blood pH
Using the aforementioned setup (Figure 1), we investigated the microcirculation in IRI over a two hours protocol; however a much longer observation time up to 6 hours is possible. As shown in Figure 2, IRI of the cremaster muscle has no significant macrohemodynamic effects on the rat circulation as mean arterial pressure and heart rate remain stable throughout the investigation period. Furthermore we monitored homeostasis by frequent measurements of the arterial blood-pH that ranged within physiological limits and showed no significant inter-group differences.
IRI induces leukocyte rolling in the cremasteric circulation
Leukocyte endothelial interaction is a key event in the acute inflammation. By means of intravital microscopy we found a time-dependent increase in the number of rolling leukocytes in IRI of the cremaster muscle (Figure 3A) concordant to previous data 21, 25. Rolling mounted over the two hours observation time to a maximum of 137.63 ± 22.55 % of the baseline value after 120 minutes of reperfusion time and then reached statistical significance compared to sham operated animals (137.63 ± 22.55 vs 99.43 ± 14.04 % of baseline value).
IRI induces leukocyte adhesion in the cremasteric circulation
For further evaluation of leukocyte-endothelial interaction we analyzed leukocyte adhesion in a 200 μm vessel section. IRI induced an increase in the number of adherent leukocytes that significantly exceeded values of sham operated animals after 60 minutes (118.33 ± 6.83 vs. 96.27 ± 5.78 % of baseline value) and further ascended by 120 minutes of reperfusion (Figure 3B).
In summary the described in vivo model supplies consistent data of acute leukocyte activation in IRI whilst animal survival and circulation stability is warranted.
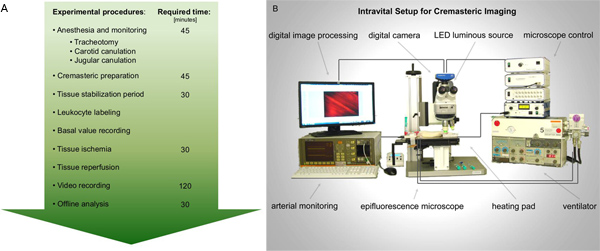
Figure 1. A. Flow chart for intravital epifluorescence microscopy performance in ischemia-reperfusion injury of the rat cremaster muscle. Following required preparations for anesthesia and monitoring, the rat cremaster muscle is exposed for imaging. Records of leukocyte activation are taken before and after tissue ischemia. Subsequent video analysis is best performed offline. B. Schematic figure on the proposed intravital setup. Click here to view larger figure.
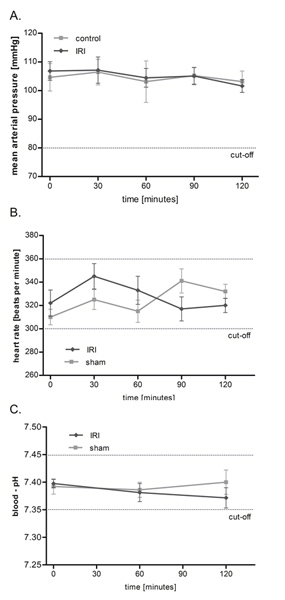
Figure 2. IRI of the cremaster muscle has no effect on mean arterial pressure (MAP), heart rate and blood pH. Mean arterial pressure (A) and heart rate (B) were monitored every 30 minutes throughout the experiment via pressure transducer after canulation of the right carotid artery. Measurement of the arterial blood-pH (C) was performed after 0, 60, and 120 minutes. Values are mean ±SEM of 6 different rats and ranged on physiological levels without significant inter-group differences. Click here to view larger figure.
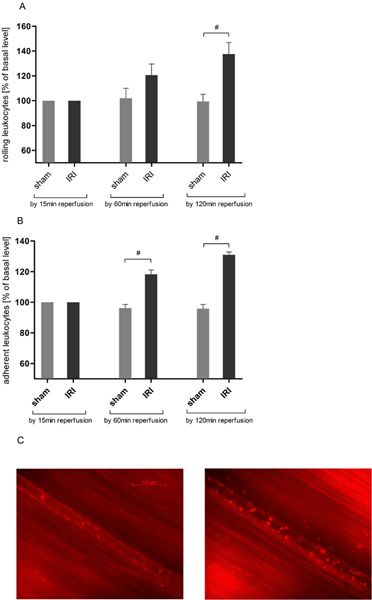
Figure 3. IRI increases leukocyte-endothelial interaction in the cremasteric circulation. After labeling of leukocytes with rhodamine 6G (0.4 mg/kg body weight) intravital epifluorescence microscopy was used to determine leukocyte-endothelial interaction in reperfusion injury over a 120 minutes protocol after 30 minutes of tissue ischemia. Records were taken at magnification of approximately ×800. A. IRI significantly increases the number of rolling leukocytes in postcapillary venules of the cremaster muscle by 120 minutes of reperfusion whereas leukocyte rolling remains stable throughout the experiment in cremasteric tissue that did not undergo ischemia. Values are mean ± SEM of 6 observed rats. # p<0.05 using the unpaired t test. B. The number of adherent leukocytes is significantly increased in a randomly chosen 200μm postcapillary vessel section after 30 minutes of cremasteric ischemia and subsequent 60 minutes of tissue reperfusion. Results get even more pronounced after a two hours reperfusion period. Values are mean ± SEM of 6 observed rats. # p<0.05 using the unpaired t test. C. Representative pictures of a postcapillary venule prior to ischemia (left) and 120 minutes after IRI (right). Click here to view larger figure.
Discussion
Leukocyte-endothelial interaction, production of reactive oxygen species and activation of the complement system are the key features of IRI-induced tissue dysfunction.26 The microcirculation of the affected tissue is considered as the integral site for the inflammatory onset. Apart from ex vivo experiments such as flow chamber assays27, 28 it is mandatory to provide well-established models of intravital imaging to further evaluate the in vivo relevance. Though IRI has been implica...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was supported by a grant of the “Deutsche Forschungsgemeinschaft” to S.U. Eisenhardt (EI 866/1-1).
Materials
| Name | Company | Catalog Number | Comments |
| Name of the equipment: | Company: | Catalogue No.: | Comments: |
| Forene 100% (V/V) | Abbot | B506 | API isoflurane |
| Terylene Suture | Serag Weissner | OC108000 | |
| Portex Fine Bore Polythene Tubing | Smiths Medical | 800/100/100 | 0.28 mm inner Diameter |
| 0,9% saline solution | Fresinus Kabi | 808771 | |
| Change-A-tip deluxe cautery kit | Bovie Medical | DEL1 | |
| Abbocath -T 14G | Venisystems | G713 - A01 | used as lens tube |
| Servo Ventilator 900C | Maquet | used as animal ventialtor | |
| Logical pressure transducer | Smiths Medical | MX1960 | |
| Sirecust 404 Monitor | Siemens | ||
| ABL 700 Benchtop Analyzer | Radiometer | for blood gas measurement | |
| Heating pad | Effenberger | 8319 | |
| Aluminum stage | Alfun | AW7022 | |
| Surgical microscope OPMI 6-SDFC | Carl Zeiss | ||
| Microsurgical instruments lab set | S&T | 767 | |
| Biemer vessel clip | Diener | 64.562 | |
| Applying forceps | Diener | 64.568 | for Biemer vessel clip |
| Rhodamine 6G | Sigma-Aldrich | R4127 | |
| Vaseline white DAB | Winthrop | 2726853 | |
| Cover glasses | 32x32 mm | ||
| Intravital setup | |||
| Zeis Axio Scope A-1 MAT | Carl Zeis | 490036 | epifluorescence microscope |
| 470 nm LED | Carl Zeis | 423052 | fluorescence light source |
| Colibri 2 System | Carl Zeis | 423052 | |
| W Plan-Apochromat 20x/1,0 DIC | Carl Zeis | 421452 | water immersion objective |
| AxioCam MRm Rev. 3 FireWire | Carl Zeis | 426509 | high resolution digital camera |
| Axio vision LE software | Carl Zeis | 410130 | use for offline analysis |
References
- Cetin, C. Protective effect of fucoidin (a neutrophil rolling inhibitor) on ischemia reperfusion injury: experimental study in rat epigastric island flaps. Ann. Plast. Surg. 47, 540-546 (2001).
- Granger, D. N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am. J. Physiol. 255, H1269-H1275 (1988).
- Lazarus, B. The role of mast cells in ischaemia-reperfusion injury in murine skeletal muscle. J Pathol. 191, 443-448 (2000).
- van den Heuvel, M. G. Review: Ischaemia-reperfusion injury in flap surgery. J. Plast. Reconstr. Aesthet. Surg. 62, 721-726 (2009).
- Rosen, S. D. Cell surface lectins in the immune system. Semin. Immunol. 5, 237-247 (1993).
- van der Flier, A., Sonnenberg, A. Function and interactions of integrins. Cell Tissue Res. 305, 285-298 (2001).
- Panes, J., Perry, M., Granger, D. N. Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. Br. J. Pharmacol. 126, 537-550 (1999).
- Gavins, F. N., Chatterjee, B. E. Intravital microscopy for the study of mouse microcirculation in anti-inflammatory drug research: focus on the mesentery and cremaster preparations. J. Pharmacol. Toxicol. Methods. 49, 1-14 (2004).
- Sutton, T. A. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am. J. Physiol. Renal. Physiol. 285, 191-198 (2003).
- Serracino-Inglott, F. Differential nitric oxide synthase expression during hepatic ischemia-reperfusion. Am. J. Surg. 185, 589-595 (2003).
- Eppinger, M. J. Mediators of ischemia-reperfusion injury of rat lung. Am J Pathol. 150, 1773-1784 (1997).
- Dumont, E. A. Real-time imaging of apoptotic cell-membrane changes at the single-cell level in the beating murine heart. Nat Med. 7, 1352-1355 (2001).
- Baez, S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res. 5, 384-394 (1973).
- Woeste, G. Octreotide attenuates impaired microcirculation in postischemic pancreatitis when administered before induction of ischemia. Transplantation. 86, 961-967 (2008).
- Schultz, J. E., Hsu, A. K., Gross, G. J. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ. Res. 78, 1100-1104 (1996).
- Dobschuetz, E. v. o. n. Dynamic intravital fluorescence microscopy--a novel method for the assessment of microvascular permeability in acute pancreatitis. Microvasc Res. 67, 55-63 (2004).
- Vutskits, L. Adverse effects of methylene blue on the central nervous system. Anesthesiology. 108, 684-692 (2008).
- Takasu, A. Improved survival time with combined early blood transfusion and fluid administration in uncontrolled hemorrhagic shock in rats. J. Trauma. 8, 312-316 (2010).
- Proctor, K. G., Busija, D. W. Relationships among arteriolar, regional, and whole organ blood flow in cremaster muscle. Am. J. Physiol. 249, 34-41 (1985).
- Bagher, P., Segal, S. S. The Mouse Cremaster Muscle Preparation for Intravital Imaging of the Microcirculation. J. Vis. Exp. (52), e2874 (2011).
- Kanwar, S., Hickey, M. J., Kubes, P. Postischemic inflammation: a role for mast cells in intestine but not in skeletal muscle. Am. J. Physiol. 275, 212-218 (1998).
- Leoni, G. Inflamed phenotype of the mesenteric microcirculation of melanocortin type 3 receptor-null mice after ischemia-reperfusion. FASEB J. 22, 4228-4238 (2008).
- Simoncini, T. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 407, 538-541 (2000).
- Woollard, K. J. Pathophysiological levels of soluble P-selectin mediate adhesion of leukocytes to the endothelium through Mac-1 activation. Circ. Res. 103, 1128-1138 (2008).
- Mori, N. Ischemia-reperfusion induced microvascular responses in LDL-receptor -/- mice. Am. J. Physiol. 276, H1647-H1654 (1999).
- Eisenhardt, S. U. Monitoring Molecular Changes Induced by Ischemia/Reperfusion in Human Free Muscle Flap Tissue Samples. Ann. Plast. Surg. , (2011).
- Eisenhardt, S. U. Generation of activation-specific human anti-{alpha}M{beta}2 single-chain antibodies as potential diagnostic tools and therapeutic agents. Blood. 109, 3521-3528 (2007).
- Eisenhardt, S. U. Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ Res. 105, 128-137 (2009).
- Eisenhardt, S. U. C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle. 8, 3885-3892 (2009).
- Granger, D. N. . Physiology and pathophysiology of leukocyte adhesion. , 520 (1995).
- Baatz, H. Kinetics of white blood cell staining by intravascular administration of rhodamine 6G. Int. J. Microcirc. Clin. Exp. 15, 85-91 (1995).
- Mempel, T. R. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr. Opin. Immunol. 16, 406-417 (2004).
- Abbitt, K. B., Rainger, G. E., Nash, G. B. Effects of fluorescent dyes on selectin and integrin-mediated stages of adhesion and migration of flowing leukocytes. J. Immunol. Methods. 239, 109-119 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved