A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Measurement of Metabolic Rate in Drosophila using Respirometry
In This Article
Summary
Metabolic disorders are among one of the most common diseases in humans. The genetically tractable model organism D. melanogaster can be used to identify novel genes that regulate metabolism. This paper describes a relatively simple method which allows studying the metabolic rate in flies by measuring their CO2 production.
Abstract
Metabolic disorders are a frequent problem affecting human health. Therefore, understanding the mechanisms that regulate metabolism is a crucial scientific task. Many disease causing genes in humans have a fly homologue, making Drosophila a good model to study signaling pathways involved in the development of different disorders. Additionally, the tractability of Drosophila simplifies genetic screens to aid in identifying novel therapeutic targets that may regulate metabolism. In order to perform such a screen a simple and fast method to identify changes in the metabolic state of flies is necessary. In general, carbon dioxide production is a good indicator of substrate oxidation and energy expenditure providing information about metabolic state. In this protocol we introduce a simple method to measure CO2 output from flies. This technique can potentially aid in the identification of genetic perturbations affecting metabolic rate.
Introduction
The biochemical Kreb's cycle generates ATP through the oxidation of acetate derived from carbohydrates, fats, and proteins producing CO2. In Drosophila, O2 input is directly correlated with CO2 output and reflects the level of metabolism1. Thus, measurement of CO2 output has successfully been used in studies related to aging and metabolism2-5. Here our laboratory has modified previously designed experimental setups, allowing measurement of CO2 production in up to eighteen samples without requiring any specialized equipment. Others and we have previously used this method to show differences in metabolic rates in flies that are deficient in the muscular dystrophy associated protein, Dystroglycan (Dg)6-8.
O2 used for oxidative metabolism is converted into CO2, which is expelled as respiratory waste. The construction of hand-made respirometers is described that allows for the determination of the rate of O2 consumed. Flies are placed in a sealed container with a substance that absorbs expelled CO2, efficiently eliminating it from the gaseous phase. The change in gas volume (decreased pressure) is measured by the displacement of fluid in a glass capillary attached to the closed respirometer.
The main advantage of this technique over others is the cost. Previous studies have measured CO2 production by Drosophila using gas analyzers and technically advanced respirometry systems1,9. Despite the more complex equipment, the sensitivity of the method described here is similar to reported values (Table 1). Additionally, several other groups have used variations of this technique to determine relative metabolic rates in Drosophila4-6. Therefore, this assay can be used to generate reliable, reproducible data relevant to Drosophila metabolism without the purchase of specialized equipment which can be setup in any lab and can be used for educational purposes.
In general, the accepted techniques to determine the metabolism of an organism is to measure the CO2 produced, the O2 consumed, or both3,4,9. Though, it can be assumed that one equivalent of O2 generates one equivalent of CO2, the precise ratio of CO2 generated is dependent on the metabolic substrate utilized10. Thus, to accurately determine the metabolic rate in energy units it is necessary to measure both O2 consumed and CO2 produced. Due to this, the method described here is specifically relevant to comparing differences in CO2 production between animals and not the absolute value. Our technique integrates multiple animal CO2 production over a period of time (1-2 hr) and therefore returns an average of the animals' activity. If there is reason to believe that the experimental animals are less active than the control animals the measurement could reflect different levels of activity and not necessarily metabolism.
Protocol
1. Preparation of Respirometers
- Cut the 1,000 μl pipette tip with a razor blade to allow insertion of the 50 μl capillary micropipette, try to get the pipette tip as straight as possible.
- Place a piece of foam into the pipette and push it down in the pipette tip.
- Add a small amount of CO2 absorbent and contain it by a second piece of foam.
- Apply glue at the place where the micropipette is inserted into the pipette tip.
- Leave the respirometer overnight to allow the glue to dry.
A schematic of a respirometer is shown in Figure 1A.
2. Preparation of the Measurement Chamber
- Prepare the chamber solution by mixing water with eosin in a ratio that will result in visible colorization.
- Pour the eosin/water solution into the chamber.
- Label one of the sides of the chamber with a centimeter scale.
3. Placing Flies into Respirometers
- Label the individual respirometers with a marker.
- Anesthetize flies using an alternative method to CO2 and place 3-5 flies of the desired genotype inside each respirometer.
- Seal the respirometers tightly at the top using plasticine putty.
- Allow flies to recover from anesthetization for approximately 15 min.
- Prepare one respirometer without flies, which will be used as the atmospheric control.
4. Performing the Experiment
- Hang the respirometers in the chamber by attaching a 1.5 ml Eppendorf tube holder that is open on the top and the bottom at the top of the chamber.
- Insert respirometers with the micropipette tip down into the chamber allowing the tip to submerge into the colored solution.
- Add Vaseline between the lid cover and the chamber to provide stronger isolation from temperature and pressure fluctuations.
- Close the lid and allow the system to equilibrate for 15 min.
- Take a photograph of the chamber making sure that the level of liquid within each micropipette is visible and so is the scale (see example shown in Figure 1B).
- After 1-2 hr, take another picture.
- When experiment is finished, remove the flies from respirometers and weigh if desired or transfer them back to the vial if needed further.
5. Analysis of Results
- Open acquired images using ImageJ software11.
- Using the scale in each picture, set the pixel scaling in the software.
- Measure the distance (Δd) that the liquid traveled from a determined reference spot in images taken at the beginning (d1) and end of experiment (d2). A schematic example is shown in Figure 1C.
- Calculate the amount of produced CO2 (µl/hr/fly) with the formula:
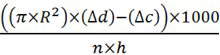
R= radius of micropipette tube in centimeters
Δd= distance the liquid has moved up in the micropipette of test samples measured in centimeters
Δc= distance the liquid has moved up in the micropipette of the negative control sample (without flies)
n = number of flies used
h= hours
Results
In order to show that the method is sensitive we measured CO2 production from wild type (Oregon R) male flies at 18, 25, and 29 °C and flies mutant for Dg. Flies were raised at 25 °C and then shifted to the experimental temperature for 5 days prior to measurement. As expected for this ectothermic species, the amount of CO2 produced increased with temperature (Figure 2). We have in the past shown that a sugar free diet reduces the metabolic rate of both wi...
Discussion
In this protocol, we describe an inexpensive and reliable method for measuring CO2 production in flies. We found that this experiment is easy, quick to conduct and generates reproducible data that is in agreement with other studies1,6,9. The protocol outlined here can be easily modified to fit any laboratory's budget and available materials. The construction of each individual respirometer can be adapted as long as the chamber remains airtight. However...
Disclosures
We have nothing to disclose.
Acknowledgements
We would like to thank Max-Planck Society for funding our research.
Materials
| Name | Company | Catalog Number | Comments |
| BlauBrand IntraMark 50 µl micropipettes | VWR | 612-1413 | |
| Soda Lime | Wako | CDN6847 | |
| Eosine | Sigma | 031M4359 | Any dye that can create visible colorization of liquid can be used |
| Thin Layer Chromatorgaphy (TLC) Developing Chamber | VWR | 21432-761 | Any transparent glass chamber that can be closed with the lid |
| Anesthetizer, Lull-A-Fly Kit | Flinn | FB1438 | |
| Power Gel Glue | Pritt | ||
| 1 ml pipett tips | Any | ||
| Foam | Any | ||
| Plaesticine Putty | Any | ||
| Scalpel | Any | ||
| Tweezers | Any |
References
- Van Voorhies, W. A., Khazaeli, A. A., Curtsinger, J. W. Testing the "rate of living" model: further evidence that longevity and metabolic rate are not inversely correlated in Drosophila melanogaster. J Appl Physiol. 97, 1915-1922 (2004).
- Ross, R. E. Age-specific decrease in aerobic efficiency associated with increase in oxygen free radical production in Drosophila melanogaster. Journal of Insect Physiology. 46, 1477-1480 (2000).
- van Voorhies, W. A., Khazaeli, A. A., Curtsinger, J. W. Selected contribution: long-lived Drosophila melanogaster. lines exhibit normal metabolic rates. J Appl Physiol. 95, 2605-2613 (2003).
- Hulbert, A. J., et al. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Experimental Gerontology. 39, 1137-1143 (2004).
- Ueno, T., Tomita, J., Kume, S., Kume, K. Dopamine modulates metabolic rate and temperature sensitivity in Drosophila melanogaster. PLoS ONE. 7, (2012).
- Takeuchi, K., et al. Changes in temperature preferences and energy homeostasis in dystroglycan mutants. Science. 323, 1740-1743 (2009).
- Kucherenko, M. M., Marrone, A. K., Rishko, V. M., Magliarelli Hde, F., Shcherbata, H. R. Stress and muscular dystrophy: a genetic screen for dystroglycan and dystrophin interactors in Drosophila. identifies cellular stress response components. Developmental Biology. 352, 228-242 (2011).
- Marrone, A. K., Kucherenko, M. M., Wiek, R., Gopfert, M. C., Shcherbata, H. R. Hyperthermic seizures and aberrant cellular homeostasis in Drosophila dystrophic. muscles. Scientific Reports. 1, 47 (2011).
- Khazaeli, A. A., Van Voorhies, W., Curtsinger, J. W. Longevity and metabolism in Drosophila melanogaster: genetic correlations between life span and age-specific metabolic rate in populations artificially selected for long life. Genetics. 169, 231-242 (2005).
- Elia, M. Energy equivalents of CO2 and their importance in assessing energy expenditure when using tracer techniques. The American Journal of Physiology. 260, (1991).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9, 676-682 (2012).
- Bharucha, K. N. The epicurean fly: using Drosophila melanogaster. to study metabolism. Pediatric Research. 65, 132-137 (2009).
- Rajan, A., Perrimon, N. Of flies and men: insights on organismal metabolism from fruit flies. BMC Biology. 11, 38 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved