A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Synthesis of Gold Nanoparticle Integrated Photo-responsive Liposomes and Measurement of Their Microbubble Cavitation upon Pulse Laser Excitation
In This Article
Summary
This protocol describes a simple preparation method for gold nanoparticle integrated photo-responsive liposomes with the commercially available materials. It also shows how to measure the microbubble cavitation process of the synthesized liposomes upon the treatment of pulsed laser.
Abstract
Photo-responsive nanoparticles (NPs) have received considerable attention because of their potential in providing spatial, temporal, and dosage control over the drug release. However, most of the relevant technologies are still in the development process and are unprocurable by clinics. Here, we describe a facile fabrication of these photo-responsive NPs with commercially available gold NPs and thermo-responsive liposomes. Calcein is used as a model drug to evaluate the encapsulation efficiency and the release kinetic profile upon heat/light stimulation. Finally, we show that this photo-triggered release is due to the membrane disruption caused by microbubble cavitation, which can be measured with hydrophone.
Introduction
The possibility to trigger drug release using external stimuli is an attractive way to deliver the drugs in spatial-, temporal- and dosage-controlled fashions with maximized specificity and minimal adverse effects. Among a wide range of exogenous stimuli-responsive systems (light, magnetic field, ultrasound, microwave radiation), light-triggered platforms are attractive, owing to their non-invasiveness, simplicity and adaptability in the clinics.1 Extensive research in the past decade has provided a variety of platform technologies, such as near-infrared-light responsible gold (Au) nanocages coated with smart polymers,2 photo-labile, polymeric nanoparticles (NPs) conjugated with drugs,3 and self-assembled porphysome nanovesicles.4 However, these technologies are still in the preclinical stages of development, and require a clear understanding and optimization of parameters involved in the process of initiating and controlling the drug release.
One of the simplest and easily accessible methods for preparing such a system is to integrate Au NPs with thermally-sensitive liposomes5,6, both of which are widely available in the market and have been extensively investigated in preclinical and even clinical trials. Despite the limitation of deep-tissue activation of Au NPs at their plasmonic wavelength, when compared to near-infrared-activated Au nanostructures (e.g., nanocages), this system still holds great promise when used in small animals or for topical delivery in humans.7 There are some early efforts in combining Au NPs with liposomes for light-triggered release.8-11 While most of them focus on the novelty of materials, accessibility and scalability issues need to be addressed. Moreover, reports on release mechanisms using these nanocarriers are still limited.
Herein, the fabrication of photo-responsive liposomes, simultaneously loaded with drugs and hydrophilic Au NPs has been described. Calcein is used as a model compound to evaluate the encapsulation efficiency and the release profile of the system. In addition, in this system, light absorbed by Au NPs dissipates to the surrounding microenvironment in the form of heat, resulting in an increase in the local temperature. Air microbubbles are generated during the laser heating and cause mechanical disruption of liposomes (Figure 1). The mechanism of microbubble cavitation is confirmed by hydrophone measurements.
Protocol
1. Preparation
- Clean 100 ml round bottom flasks using aqua regia (1 part of concentrated nitric acid (HNO3) and 3 parts of concentrated hydrochloric acid (HCl)) and wash the flasks with DI water. Autoclave the flasks and dry them in a hot air oven at 100 °C for 15 min. Wrap and store the sterile flasks until use.
- Sterilize the hand-held mini-extruder set using 70% ethanol.
- Turn on the rotary evaporator and set the temperature of the hot water bath and the cooling tower at 37 °C and 4 °C respectively.
- Prepare 60 mM calcein stock solution by dissolving 374 mg of calcein in 10 ml of 0.1 mM phosphate buffered saline (PBS) (pH 7.4). Adjust the pH to 7.4 using 1 M sodium hydroxide (NaOH) solution.
2. Synthesis of Liposomes
- Remove the lipids (1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (MPPC) and 1, 2-distearoyl-sn-glycero-3-phosphoethanol-amine-N-[carboxy(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG2000)) from freezer (-20 °C) and thaw them to RT.
- Weigh 15.9 mg DPPC, 1.3 mg MPPC, and 2.8 mg DSPE-PEG2000. Dissolve them together in 2 ml chloroform.
- Transfer the chloroform solution to the sterile round bottom flask and evaporate the solvent using the rotary evaporator under reduced pressure, to form a thin, dry lipid layer.
- Hydrate the lipid layer at 45 °C with 2 ml aqueous solution for 30 min containing 1.95 ml 60 mM calcein prepared in step 1.4 and 50 µl Au NPs (3.36 × 1016 particles/ml).
- Pre-heat the heating block to 45 °C. Place a 200 nm polycarbonate membrane filter between the filter supports and assemble the mini-extruder set. Check the potential leakage with DI water.
- Fill one of the syringes with 1 ml of liposome solution from step 2.4 and extrude the sample by passing the solution to the syringe at the other end of the assemble mini-extruder. Repeat 11 times.
- Run the synthesized liposomes through a PD-10 desalting column to remove the free Au NPs, lipids and calcein using PBS as the eluent according to manufacturer's protocol.
- Store the samples in sterile tubes at 4 °C and use in 2 days.
3. Calcein Release from Liposomes with Heating
- Calculate the lipid molarity of the stock solution by adding up the molarities of DPPC, MPPC, and DSPE-PEG2000 in step 2.2. Dilute the liposome stock solution to 5 mM lipid concentration using 0.1 mM PBS buffer (pH 7.4). Transfer the samples to a centrifuge tube (2 ml).
- Place the tube in a hot water bath, and raise the temperature gradually from 25 to 70 °C. Increase the temperature at a rate of 1 °C/min here.
- Collect aliquots (10 µl) at different temperature points (27, 32, 37, 39, 41, 43, 45, 52, 57, 62, 67 and 70 °C).
- Add 10 µl of 2% Triton X-100 to 2 ml aliquot of liposomal solution to digest the liposomes for 10 min at RT and to achieve complete release of calcein.
- Transfer 200 µl of the liposomal solution to each well in the 96-well microplate and measure the fluorescence intensity of the collected samples using a fluorescence microplate reader. The excitation and emission wavelengths of calcein are 480 and 515 nm respectively.
- Taking the fluorescence intensity of the Triton X-100 treated samples as 100% release, calculate the percentage of the released calcein at each time point, using the formula:
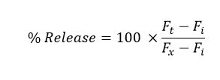
Ft is the fluorescence intensity of solution at a given time point. Fi and Fx are the normalized initial and final fluorescence intensities of the solution respectively.
4. Calcein Release from Liposomes with Pulsed Laser
- Transfer 100 µl liposome solution to a quartz cuvette and place it in a cuvette holder. Use a pulsed Nd:YAG laser with a pulse duration of 6 nsec at 532 nm wavelength as the light source. Use the following Laser Parameters — Repetition rate: 1 Hz; Laser energy density: 1 mJ/cm2; Beam diameter: 0.5 mm.
- Guide the collimated laser beam through the cuvette such that the light passes through the liposome solution and collect aliquots after various pulses.
- Add 10 µl of 2% Triton X-100 to 2 ml aliquot of liposomal solution to digest the liposomes for 10 min at RT and to achieve complete release of calcein.
- Considering calcein is sensitive to light and could be bleached during the laser experiment, pre-measure the bleaching effect of pulsed laser on calcein solution to normalize the data from liposome release.
- Specifically, expose calcein solution (60 mM) to pulsed laser with frequency of 1 Hz for varying pulse numbers (0, 25, 50 and 100). Measure the fluorescence intensity of calcein with excitation and emission wavelengths of 480 and 515 nm respectively, before and after laser exposure.
- Calculate the amount of bleaching (fluorescence intensity of calcein before laser exposure/ fluorescence intensity of calcein after the specific pulse number). Use this factor to normalize the values obtained from liposome samples by multiplying the fluorescence intensity of the liposomes with the factor.
- Measure the fluorescence intensity of the collected sample using a fluorescence microplate reader at excitation and emission wavelengths at 480 and 515 nm respectively.
- Taking the fluorescence intensity of the Triton X-100 treated samples as 100% release, calculate the percentage of the released calcein at each pulse number, using the formula:
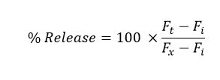
Ft is the fluorescence intensity of solution at a given pulse number after normalization. Fi and Fx are the normalized initial and final fluorescence intensities of the solution respectively.
5. Measurement of Pressure Impulses
- Place 100 µl of the sample on a microscopic slide and set the focus of the laser onto the sample.
- Immerse a needle hydrophone (1 mm diameter and 450 nV/Pa sensitivity) into the solution.
Caution: The hydrophone must not be illuminated by the laser light to avoid the damage. - Irradiate the sample with the pulsed laser with varying pulse numbers (0-100) and pulse energy (20-160 µJ/pulse).
- Record the pressure signals using a digital oscilloscope.
Results
Liposomes were prepared using a conventional thin film hydration technique with DPPC, MPPC and DSPE-PEG2000 in a molar ratio of 86:10:4 or 7.95:0.65:1.39 mg/ml.12 The size of Au NPs is critical to determine the light to heat conversion efficiency during the following laser excitation experiment. Smaller the size of Au NPs, higher is the transducing efficiency.13 Thus 5 nm Au NPs, the smallest samples from the vendor, were chosen for encapsulation. During the synthesi...
Discussion
Thin film hydration is the conventional method for preparing liposomes. Organic solvents (chloroform in this case) were first used to dissolve the lipids and then removed in a rotary evaporator at 37 °C to generate a lipid thin film on the flask. This lipid film was hydrated with the aqueous solution containing 60 mM calcein and 5 nm Au NPs. During the hydration process, the temperature was maintained around 50 °C and the flask was constantly agitated by rotating the flask. The key in this step is the choice of...
Disclosures
No conflict of interest are declared.
Acknowledgements
This work was partially supported by the Tier-1 Academic Research Funds by Singapore Ministry of Education (RG 64/12 to CX) and NTU-Northwestern Institute of Nanomedicine.
Materials
| Name | Company | Catalog Number | Comments |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) | Avanti Polar Lipids (Alabama, US) | 850355P | Powder, Store at -20 °C |
| 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (MPPC) | Avanti Polar Lipids (Alabama, US) | 855675P | Powder, Store at -20 °C |
| 1,2-distearoyl-sn-glycero-3-phosphoethanol-amine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG2000) | Avanti Polar Lipids (Alabama, US) | 880120P | Powder, Store at -20 °C |
| Gold Nanoparticles | Sigma Aldrich | 752568-100mL | 5 nm particles, stabilized at 0.1 mM PBS |
| Calcein | Sigma Aldrich | C0875-10g | 60 mM, pH 7.4 (adjusted using NaOH) |
| phosphate buffered saline (PBS) | Sigma Aldrich | P5493 | 0.1 mM, pH 7.4 |
| Double distilled water | Millipore Milli-DI water purification system | ||
| Triton X100 | Sigma, Life Sciences | X-100 | To disrupt the liposomes to calculate total encapsulation |
| Rotavapor | Buchi (Switzerland) | R 210 | Used for Lipososme preparation |
| Heating bath | Buchi (Switzerland) | B 491 | Used for Lipososme preparation |
| Vacuum Controller | Buchi (Switzerland) | V-850 | Used for Lipososme preparation |
| Vacuum Pump | Buchi (Switzerland) | V-700 | Used for Lipososme preparation |
| Recirculation bath with temperature controller | Polyscience | Used for Lipososme preparation | |
| Mini-extruder assembly with heating block | Avanti Polar Lipids (Alabama, US) | 610000 | Used for extrusion of liposomes |
| Syringes, 1,000 μl | Avanti Polar Lipids (Alabama, US) | 610017 | Used for extrusion of liposomes |
| Polycarbonate filter membrane, 200 nm | Whatmann | 800281 | Used for extrusion of liposomes |
| Filter Support | Avanti Polar Lipids (Alabama, US) | 610014 | Used for extrusion of liposomes |
| PD 10 Desalting coulumns, Sephadex G-25 medium | GE Healthcare, Life sciences | 17-0851-01 | Used to purify the liposomes |
| Centrifuge | Sigma Laboratory Centrifuges | 3K30 | Used to concentrate the liposomal solution |
| Rotor | Sigma | 19777-H | Used to concentrate the liposomal solution |
| Zetasizer | Nano ZS Malvern | Used for the determination of liposome size and zetapotential | |
| UV-Visible Spectrophotometer | Shimadzu | UV-2450 | Used to measure the absorbance of the samples |
| Fluorescent Spectrofluorometer | Molecular Devices | SpectraMax M5 | Used to measure the fluorescence emission of the samples |
| Nd:YAG Laser | NewWave Research | 532 nm; Maximum power: 17 mJ; Width: 406 nsec; Used for sample irradiation | |
| HNR Hydrophone | ONDA | HNR-1000 | 1 mm diameter and 450 nV/Pa sensitivity, Proper working frequency range: 0.25-10 MHz; Calibration: 50 mV/Bar; Used to measure the acoustic signals |
| Digital Osciloscope | LECORY - Wave Runner 64Xi-A | Frequency: 600 MHz; Max sample rate: 10 Gs/sec (at two channel); Used to record the measured acoustic signals |
References
- McCoy, C. P., et al. Triggered drug delivery from biomaterials. Expert Opin. Drug Deliv. 7 (5), 605-616 (2010).
- Yavuz, M. S., et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 8 (12), 935-939 (2009).
- Gohy, J. F., Zhao, Y. Photo-responsive block copolymer micelles: design and behavior. Chem. Soc. Rev. 42 (17), 7117-7129 (2013).
- Lovell, J. F., et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 10 (4), 324-332 (2011).
- Needham, D., Dewhirst, M. W. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Deliv. Rev. 53 (3), 285-305 (2001).
- Landon, C. D., Park, J. Y., Needham, D., Dewhirst, M. W. Nanoscale drug delivery and hyperthermia: the materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed. J. 3, 38-64 (2011).
- Sykes, E. A., Dai, Q., Tsoi, K. M., Hwang, D. M., Chan, W. C. Nanoparticle exposure in animals can be visualized in the skin and analysed via skin biopsy. Nat. Commun. 5, 3796 (2014).
- Paasonen, L., et al. Gold nanoparticles enable selective light-induced contents release from liposomes. J. Control. Release. 122 (1), 86-93 (2007).
- Wu, G., et al. Remotely Triggered Liposome Release by Near-Infrared Light Absorption via Hollow Gold Nanoshells. J. Am. Chem. Soc. 130 (26), 8175-8177 (2008).
- Leung, S. J., Kachur, X. M., Bobnick, M. C., Romanowski, M. Wavelength-Selective Light-Induced Release from Plasmon Resonant Liposomes. Adv. Funct. Mater. 21 (6), 1113-1121 (2011).
- Volodkin, D. V., Skirtach, A. G., Möhwald, H. Near-IR Remote Release from Assemblies of Liposomes and Nanoparticles. Angew. Chem. Int. Ed. 48 (10), 1807-1809 (2009).
- Mills, J. K., Needham, D. Lysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transition. Biochim. Biophys. Acta. 1716 (2), 77-96 (2005).
- Jiang, K., Smith, D. A., Pinchuk, A. O. Size-dependent Photothermal Conversion Efficiencies of Plasmonically Heated Gold Nanoparticles. J. Phys. Chem. C. 117 (51), 27073-27080 (2013).
- Chongsiriwatana, N., Barron, A., Giuliani, A., Rinaldi, A. C. Comparing bacterial membrane interaction of antimicrobial peptides and their mimics. Antimicrobial Peptides. 618, 171-182 (2010).
- Egerev, S., et al. Acoustic signals generated by laser-irradiated metal nanoparticles. Appl. Opt. 48 (7), C38-C45 (2009).
- González, M. G., Liu, X., Niessner, R., Haisch, C. Strong size-dependent photoacoustic effect on gold nanoparticles by laser-induced nanobubbles. Appl. Phys. Lett. 96, 174104 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved