A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Development and Maintenance of a Preclinical Patient Derived Tumor Xenograft Model for the Investigation of Novel Anti-Cancer Therapies
In This Article
Summary
Utilizing patient-derived tumors in a subcutaneous preclinical model is an excellent way to study the efficacy of novel therapies, predictive biomarker discovery, and drug resistant pathways. This model, in the drug development process, is essential in determining the fate of many novel anti-cancer therapies prior to clinical investigation.
Abstract
Patient derived tumor xenograft (PDTX) models provide a necessary platform in facilitating anti-cancer drug development prior to human trials. Human tumor pieces are injected subcutaneously into athymic nude mice (immunocompromised, T cell deficient) to create a bank of tumors and subsequently are passaged into different generations of mice in order to maintain these tumors from patients. Importantly, cellular heterogeneity of the original tumor is closely emulated in this model, which provides a more clinically relevant model for evaluation of drug efficacy studies (single agent and combination), biomarker analysis, resistant pathways and cancer stem cell biology. Some limitations of the PDTX model include the replacement of the human stroma with mouse stroma after the first generation in mice, inability to investigate treatment effects on metastasis due to the subcutaneous injections of the tumors, and the lack of evaluation of immunotherapies due to the use of immunocompromised mice. However, even with these limitations, the PDTX model provides a powerful preclinical platform in the drug discovery process.
Introduction
Colorectal cancer (CRC) is a significant contributor to cancer deaths in the United States. In 2015, there were an estimated 132,700 new cases of CRC with 49,700 deaths 1. Although the prognosis in patients with localized disease is excellent, patients with advanced disease have poor outcomes, making this a major priority in the development of novel therapies. Despite standard of care chemotherapeutic regimens and newer biologics that are deployed against this disease, there has been only an incremental increase in overall survival. Accordingly, there is a significant effort in understanding the driver pathways involved in facilitating tumor growth in this disease. The Cancer Genome Atlas Network has recently identified numerous main pathways that are implicated in CRC dysregulation and include: WNT, phosphoinositide 3-kinase (PI3K), RAS, transforming growth factor-β (TGF- β) and TP53 2. Together, with investigations describing other pathways that potentiate growth in CRC have ignited the development of newer therapies aimed at significantly improving the survival in this patient population 3-5. Utilizing preclinical models in oncology drug development have been essential in this process in predicting the clinical activity of these novel compounds.
Various preclinical models have been utilized in the drug development process. Considering that preclinical transgenic animal models and immortalized cell lines have been unsuccessful in determining the clinical activity of novel oncology therapies, largely due to their inability to reflect the complexity of human tumors, patient-derived tumor xenograft (PDTX) models have been established. The greatest advantage of this model is that tumor heterogeneity remains intact and closely reflects the molecular characteristics and clonality of the originating patient tumor 6-9. PDTX models provide an excellent in vivo preclinical platform to study novel agents, drug resistance pathways, combinational strategies, and cancer stem cell biology 10.
A general overview of the PDTX process is illustrated in Figure 1. It begins in the clinic, consenting patients to allow some of their excess tumor tissue to be used for this research. Next, at surgery, a piece of the tumor is grossed by a pathologist and put into media to be transported to research personnel. Immediately after this, a section of the tumor is cut into small pieces and transplanted into immunodeficient mice subcutaneously. Once the tumor grows, it is passaged into different generations of mice in order to maintain the tumor10. Typically, after the F3 generation the tumor can be expanded into a treatment study where novel compounds and/or combinational therapies are evaluated. Utilizing Next Gen Seq (Exome Seq, RNA Seq and SNP array) potential predictive biomarkers are discovered that assist in the selection of patients that may derive benefit from a particular treatment.
The overarching goals of using PDTX models are to: 1) evaluate the efficacy of novel therapies as single agent or in combination and 2) identify predictive biomarkers of sensitivity or resistance prior to clinical investigation. In this manuscript, we provide the methodology in the initiation and maintenance of a CRC PDTX bank and provide the advantages and limitations of this model in drug development discovery.
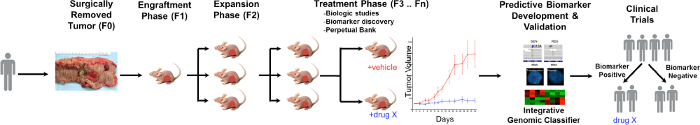
Figure 1. Overview of the CRC PDTX Model Protocol. A patient derived tumor is received from surgery and immediately injected into athymic nude mice subcutaneously. Once the tumor grows it is expanded into subsequent generations and eventually expanded for treatment studies. Treatment responses are assessed and predictive biomarkers are identified that may aid in patient selection. Please click here to view a larger version of this figure.
Protocol
Ethics Statement: Patient-derived colorectal adenocarcinoma tumor specimens were obtained from consenting patients at the University of Colorado Hospital in accordance with a protocol approved by the Colorado Multiple Institutional Review Board (08-0439). All animal work was performed under animal protocols approved by the University of Colorado Denver Institutional Animal Care and Use Committee (IACUC, Protocol # 51412(06)1E and 96813(04)1E).
1. Receiving and Preparing Patient Blood
- Collect 1 - 2 ml of blood in a blood/cell separation tube containing sodium citrate (tube phases included are plasma, lymphocyte and monocyte band, density gradient fluid, gel barrier, and erythrocytes and neutrophils). Caution, follow Blood Borne Pathogen guidelines with blood or tissue.
Note: PBMCs and plasma could be used for future studies that may include: comparing germline genetic variation with tumor mutations, isolating circulating tumor cells, evaluating cell free DNA (cfDNA), examining proteins and microRNAs, etc. - Centrifuge the blood/cell separation tube at 2,500 x g for 15 min with no brake at RT.
- Collect peripheral blood mononuclear cells (PBMCs in lymphocyte and monocyte band of tube) and put into a 1.5 ml microcentrifuge tube (clear, autoclavable, DNase, RNase, and pyrogen free), and fill to the top of the tube with sterile Phosphate-Buffered Saline (PBS).
- Centrifuge at 2,300 x g for 3 min at RT to form a pellet of PBMCs at the bottom of the tube. Next, remove the supernatant carefully. Add 1 ml of sterile PBS to wash the pellet and centrifuge at 3,000 x g for 30 sec and then remove supernatant.
- Use a pipette to collect plasma from the blood/cell separation tube (in plasma phase of tube) in a 1.5 ml sterile cryogenic vial (DNase and RNase free, no human DNA, endotoxin free) and put the plasma and pellet of PMBC's into a -80 °C freezer for storage.
2. Receiving and Preparing Patient Tumor Sample
- Prepare either RPMI or DMEM media with 1:100 (10 %) of Penicillin-Streptomycin and Non-essential Amino Acids, 1:1000 (1%) Plasmocin, and 10 % inactivated Fetal Bovine Serum (complete media) and add 20 - 25 ml to a sterile collection cup for the tumor specimen.
- Retrieve tumor sample in a sterile cup containing complete media on ice or keep at 4 °C.
Note: The tumor sample is a piece of excess patient tumor tissue that is removed by a surgeon, processed by a pathologist, and placed into a sterile collection cup with complete media. Caution, follow Blood Borne Pathogen guidelines with blood or tissue. Also, ideally to inject five mice, the tumor should be approximately 1 cm³. - Bring the tumor sample to the animal facility for injecting into immunocompromised mice.
Note: It is best to process the tumor sample as soon as possible.- In a laminar flow hood, place the tumor sample into a sterile plastic cutting dish from the tumor cup. Use autoclaved-small scissors and forceps to cut into 10 - 12 approximately 3 x 3 x 3 mm pieces and place into an autoclaved 1.5 ml microcentrifuge tube filled with 300 µl of gelatinous protein mixture solution. Keep tube on ice.
- As a priority inject tumor into mice, but if there is any tumor left over, collect flash frozen vials (FF), formalin fixed paraffin embedded (FFPE) cups, and a viable tumor vial.
- For FF, collect small pieces of tumor tissues from a sterile cryogenic vial for further analysis such as protein, RNA, or DNA isolation. Place FF immediately into a liquid nitrogen dewar and store long-term in a -80 °C freezer.
- For FFPE, if there is enough tumor, place 3 small pieces into a 10 ml 10 % formalin cup and process into paraffin embedded blocks once tumor has been in formalin for at least 24 hr.
Note: 24 hr is based off our Histology Core suggestions 11. - Lastly, place the remaining tumor into a cryogenic tube. Make viable media by adding 10 % Dimethyl Sulfoxide (DMSO) to complete media. Next, add 1 ml to a cryogenic tube and store on ice. Note: Do not keep on ice for long periods of time.
- Cut any leftover tumor into small pieces that ideally will be enough for 10 tumors to be injected into 5 mice in the future. Then, place the viable tumor tube on ice until it can be placed into an Isopropyl Alcohol freezing container (slowly freezes the tumor 1°C at a time) and put into -80 °C freezer to ensure that the tumors do not die during the freezing process.
Note: For long-term storage the tumors are removed (after 2 - 3 days) and placed into a large liquid nitrogen dewar. Please note that viable tubes should not be allowed to thaw unless it is being taken out to inject into mice.
- Cut any leftover tumor into small pieces that ideally will be enough for 10 tumors to be injected into 5 mice in the future. Then, place the viable tumor tube on ice until it can be placed into an Isopropyl Alcohol freezing container (slowly freezes the tumor 1°C at a time) and put into -80 °C freezer to ensure that the tumors do not die during the freezing process.
3. Injection of Patient Derived Tumor Xenografts
- Use five 6 - 8 week old male and/or female athymic nude mice (T-cell deficient) for each separate patient derived tumor. Inject a total of 10 tumors (2 injections per mouse) subcutaneously (SQ).
Note: The original human tumor is designated as F0 and then once injected into mice the next generation is F1. Also,use autoclaved forceps, scissors, and trocars for each unique explant. - Load pieces of tumor from the gelatinous protein mixture solution into autoclaved 12 G trocars and ensure that the tumor is completely pushed into the trocar.
- In a sterile laminar flow hood, place 5 mice into an anesthesia box that is connected to an Isoflurane anesthesia machine (started at the initial rate of 5% Isoflurane, 3 - 4% oxygen, and 2 - 3% Isoflurane for maintenance of anesthesia) and charcoal filter (absorbs excess gas).
Note: An experienced technician should be able to perform the entire procedure on a cage of 5 mice within approximately 5 min total (roughly 30 sec per mouse). Therefore additional thermal support and eye lubrication is not generally used. For less experienced individuals or during training, it is highly recommended that individuals either perform the procedure on less animals at one time and/or use a warming pad/eye lubrication during procedure if animals will be anesthetized longer than 5 min. Procedure is based upon IACUC standards at our university.
- In a sterile laminar flow hood, place 5 mice into an anesthesia box that is connected to an Isoflurane anesthesia machine (started at the initial rate of 5% Isoflurane, 3 - 4% oxygen, and 2 - 3% Isoflurane for maintenance of anesthesia) and charcoal filter (absorbs excess gas).
- Pinch a mouse toe to ensure the mouse is no longer responsive, then take the mouse out of the Isoflurane box. Place the mouse onto a clean field to inject tumors on the middle dorsal neck region and sliding the trocar down subcutaneously until the flank region is reached with autoclaved 12 G trocars.
- Deliver one tumor subcutaneously to each side of the mouse's flank. Pinch the tumor when the trocar is pulled out, ensuring that the tumor stays in the desired flank region. The gelatinous protein mixture hardens with the mouse’s body temperature and encapsulates the tumor for approximately 1 week to help the tumor grow and secure it to the SQ connective tissue. The lesion made by the trocar is no larger than 4 mm and there is no need to close the skin with staples.
Note: Our veterinarians determined no closure is needed based on very small size of the lesion, quick healing time (middle dorsal neck region reduces any skin tension or grooming of lesion), no infections noted, and close monitoring of the animals during this time period.
- Deliver one tumor subcutaneously to each side of the mouse's flank. Pinch the tumor when the trocar is pulled out, ensuring that the tumor stays in the desired flank region. The gelatinous protein mixture hardens with the mouse’s body temperature and encapsulates the tumor for approximately 1 week to help the tumor grow and secure it to the SQ connective tissue. The lesion made by the trocar is no larger than 4 mm and there is no need to close the skin with staples.
- Before the mouse is fully awake inject Meloxicam 2 mg/kg SQ (pain medication) away from the site of tumor injection. Next, place the mouse into the cage and monitor until the mouse is awake and moving.
Note: Do not leave recovering animals unattended until fully awake. Meloxicam dosage is based upon IACUC standards at our university.- Next, repeat the same procedure with the 4 remaining mice and monitor their breathing.
Note: Please note this is a very quick procedure and mice wake up very soon after Meloxicam injection, but adjust Isoflurane as needed. Each time a mouse is taken out, turn down the Isoflurane. The Meloxicam will last 24 hr and the lesions from the trocars will fully heal in 1 week.
- Next, repeat the same procedure with the 4 remaining mice and monitor their breathing.
4. Maintenance of Patient Derived Tumor Xenograft Bank
- Monitor growth and health of mice at least once per week. Use a spreadsheet (or other tracking system) to track tumor sizes, tumor generation, date of tumor injection, and health of mice.
Note: If mice have lost 15% of their original body weight, low body condition scores (≥ 2), have tumor ulcerations, tumors reaching 2,000 mm3 or total tumors 3,000 mm3, or are sickly (hunched, cold, lethargy, etc.) in any way, mice are euthanized via CO₂ or anesthetized followed by cervical dislocation as a secondary method. Euthanize via anesthesia and cervical dislocation if collecting tumor, otherwise mice are euthanized via CO₂ and cervical dislocation. - When a tumor is approximately 1,500-2,000 mm3 anesthetize the mice as described above and then perform cervical dislocation to euthanize.
- Check that the mouse has no heartbeat. Excise the SQ tumor with autoclaved scissors and forceps.
NOTE: Allow tumors to grow for up to 1 year and if no tumor is seen then euthanize the mice via CO2, followed by cervical dislocation as a secondary method. - Passage the best growing tumor into the next generation (aka a new set of 5 mice). Use instructions above to collect 10 - 12 tumors for passing and then collect the leftover tumor as also described above.
Note: Collecting numerous viable tubes is very important in the early generations (F1 - F8); therefore collect several viable tubes, FF tubes, and 1 FFPE per generation. - Keep the remaining mice until a new generation of mice has tumor growth of approximately 300 mm3. When remaining mice have tumors that are large, continue to collect as described above.
- Check that the mouse has no heartbeat. Excise the SQ tumor with autoclaved scissors and forceps.
- Continue to passage tumors and collect at each stage until F15. At this point, take a viable tube of the earliest generation possible out of liquid nitrogen and let thaw on ice and follow tumor-injecting procedures described above.
Note: Tumors that grow from viable tubes take longer to grow in mice compared to passing from generation to generation, so keep that in mind when planning. If a particular PDTX model is no longer needed at any passage, euthanize and collect tumor, to ensure numerous viable tubes are collected for future use.
5. Developmental Therapeutics with Patient Derived Tumor Xenografts
Note: Most tumors at F3 generation have good growth kinetics (grow faster and more consistent), therefore, proceed to PDTX drug efficacy studies.
- When the desired tumor is very large (1,500 - 2,000 mm3), follow procedures above to collect tumor to expand into desired amount of mice.
- Depending on the hypothesis being tested determine the number of tumors needed per treatment group.
Note: 1 ml of gelatinous protein mixture solution can approximately fit 30 small tumor pieces (depending on tumor morphology) for injecting into mice.
- Depending on the hypothesis being tested determine the number of tumors needed per treatment group.
- Check mice with tumors weekly and when most tumors are visibly small (approximately between 50 - 300 mm³) measure the tumors with calipers to determine the tumor volume. Tumor volume = [width² (smallest measurement) x length (largest measurement)] x 0.52.
Note: The gelatinous protein mixture solution surrounds the injected tumor pieces for one week, then after one-week tumor growth will be accurate. - Use tumor volumes between 50 - 300 mm³ and then average the left and right tumors. Then randomize into treatment groups with 10 tumors per group and a group average within a few numbers of each other. Next, sort the mice into groups and begin the desired treatment study.
- Dose the mice with drug (schedule dependent on drug), weigh and measure (tumor) twice per week. At the end of study, euthanize mice via anesthesia and cervical dislocation and collect tumor for future pharmacodynamic analysis in the lab.
Note: The study lasts for 30 days depending on vehicle tumor size and health of mice.
6. Organization of a PDTX bank
- Keep a well-documented form to prevent repeating of research and appropriate use of animals.
NOTE: This is key to a successful PDTX bank.- Use spreadsheets to keep track of a tumor from the human patient in the clinic, to mice in the PDTX bank, treatments, data, and what is collected. Note: this includes freezer, liquid nitrogen dewars, and FFPE storage as well.
Results
Similarities of Common Mutations in the CRC PDTX Models and the TCGA
We investigated whether the percentage of common mutations (KRAS, NRAS, BRAF, PIK3CA, APC, CTNNB1 and TP53) in the CRC PDTX bank were representative to the mutation frequency seen in the CRC patient population. As shown in Figure 2A (TCGA) and B (CRC PDTX bank), the frequency of mutations in these gene...
Discussion
The PDTX drug discovery platform offers an improved model to the shortcomings of other preclinical models that are unreliable in predicting clinical activity of novel compounds. Importantly, tumors in this model are biologically stable, retain metastatic potential, and exhibit similar drug responsiveness from generation to generation. In this model, patient derived tumors are injected into athymic nude mice, passaged, and subsequently used in therapeutic evaluation. There are several critical steps for a successful PDTX ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by grant 1R01CA152303-01.
Materials
| Name | Company | Catalog Number | Comments |
| RPMI or DMEM | Corning | 10-040-CV | |
| Penicillin-Streptomycin | Corning | 30-002-CI | |
| Non-essential Amino Acids | Corning | 25-025-CI | |
| Fetal Bovine Serum | Corning | 35-010-CV | Thaw in -4 °C, then activate for 30 min at 60 °C water bath |
| CPT blood tube | BD vacutainer | 362761 | |
| Microcentrifuge tube | Surelock | A-7002 | |
| Phosphate-Buffered Saline | Corning | 21-040-CV | |
| Cyrogenic vials | Cyroking | C0732901 | |
| Plastic tumor cutting dish | Trueline | TR4001 | |
| Scissors | Roboz | RS-5881 | |
| Forceps | Roboz | RS-5135 | |
| Matrigel (gelatinous protein mixture) | Corning | 354234 | Store at -20 or -80 °C, then thaw on ice, do not leave at RT |
| 10% Formalin cups | Protocol | 032-059 | |
| Liquid Nitrogen Dewar Storage | Thermolyne | CY50900 | |
| Portable liquid nitrogen dewar | Nalgene | 4150-2000 | |
| Dimethyl Sulfoxide | Fischer | 67-68-5 | |
| Freezing container: Mr Frosty | Nalgene | 5100-0001 | |
| Isopropyl Alcohol | Decon | 64-17-5 | |
| Trocars | Innovative Research of America | MP-182 | |
| Anesthesia machine | Patterson Veterinary | ||
| Anesthesia box | Patterson Veterinary | ||
| Isoflurane | Vet one | 1038005 | |
| F-Air Canister | Bickford Omnicon | 80120 | |
| Meloxicam | Vet one | 5182-90C | |
| Calipers | Fowler | 54-100-167 | |
| Weight scale | Ohaus | Scout Pro SP601 |
References
- Siegel, R. L., Miller, K. D., Jemal, A. Cancer statistics. 2015. CA Cancer J Clin. 65 (1), 5-29 (2015).
- . Comprehensive molecular characterization of human colon and rectal cancer. Nature. 487 (7407), 330-337 (2012).
- Arcaroli, J. J., et al. Tumours with elevated levels of the Notch and Wnt pathways exhibit efficacy to PF-03084014, a gamma-secretase inhibitor, in a preclinical colorectal explant model. Br J Cancer. 109 (3), 667-675 (2013).
- Hubbard, J., Grothey, A. Antiangiogenesis agents in colorectal cancer. Curr Opin Oncol. 22 (4), 374-380 (2010).
- van Es, J. H., et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 435 (7044), 959-963 (2005).
- Cassidy, J. W., Caldas, C., Bruna, A. Maintaining Tumor Heterogeneity in Patient-Derived Tumor Xenografts. Cancer Res. 75 (15), 2963-2968 (2015).
- Jin, K., et al. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 12 (7), 473-480 (2010).
- Julien, S., et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 18 (19), 5314-5328 (2012).
- Siolas, D., Hannon, G. J. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 73 (17), 5315-5319 (2013).
- Tentler, J. J., et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 9 (6), 338-350 (2012).
- Carson, F. L. . Histotechnology: A Self-Assessment Workbook. , (1996).
- Arcaroli, J. J., et al. Common PIK3CA mutants and a novel 3' UTR mutation are associated with increased sensitivity to saracatinib. Clin Cancer Res. 18 (9), 2704-2714 (2012).
- Arcaroli, J. J., et al. A NOTCH1 gene copy number gain is a prognostic indicator of worse survival and a predictive biomarker to a Notch1 targeting antibody in colorectal cancer. Int J Cancer. 138 (1), 195-205 (2016).
- Arcaroli, J. J., et al. Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer. Clin Cancer Res. 16 (16), 4165-4177 (2010).
- Lieu, C. H., et al. Antitumor activity of a potent MEK inhibitor, TAK-733, against colorectal cancer cell lines and patient derived xenografts. Oncotarget. 6 (33), 34561-34572 (2015).
- Pitts, T. M., et al. Association of the epithelial-to-mesenchymal transition phenotype with responsiveness to the p21-activated kinase inhibitor, PF-3758309, in colon cancer models. Front Pharmacol. 4, 35 (2013).
- Song, E. K., et al. Potent antitumor activity of cabozantinib, a c-MET and VEGFR2 inhibitor, in a colorectal cancer patient-derived tumor explant model. Int J Cancer. 136 (8), 1967-1975 (2015).
- Tentler, J. J., et al. Identification of predictive markers of response to the MEK1/2 inhibitor selumetinib (AZD6244) in K-ras-mutated colorectal cancer. Mol Cancer Ther. 9 (12), 3351-3362 (2010).
- Bardelli, A., et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 3 (6), 658-673 (2013).
- Bertotti, A., et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 1 (6), 508-523 (2011).
- Davis, S. L., et al. Combined inhibition of MEK and Aurora A kinase in KRAS/PIK3CA double-mutant colorectal cancer models. Front Pharmacol. 6, 120 (2015).
- Morelli, M. P., et al. Preclinical activity of the rational combination of selumetinib (AZD6244) in combination with vorinostat in KRAS-mutant colorectal cancer models. Clin Cancer Res. 18 (4), 1051-1062 (2012).
- Pitts, T. M., et al. Dual pharmacological targeting of the MAP kinase and PI3K/mTOR pathway in preclinical models of colorectal cancer. PLoS One. 9 (11), e113037 (2014).
- Spreafico, A., et al. Rational combination of a MEK inhibitor, selumetinib, and the Wnt/calcium pathway modulator, cyclosporin A, in preclinical models of colorectal cancer. Clin Cancer Res. 19 (15), 4149-4162 (2013).
- Arcaroli, J. J., et al. ALDH+ tumor-initiating cells exhibiting gain in NOTCH1 gene copy number have enhanced regrowth sensitivity to a gamma-secretase inhibitor and irinotecan in colorectal cancer. Mol Oncol. 6 (3), 370-381 (2012).
- Hoey, T., et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 5 (2), 168-177 (2009).
- Ikebuchi, F., et al. Dissociation of c-Met phosphotyrosine sites in human cells in response to mouse hepatocyte growth factor but not human hepatocyte growth factor: the possible roles of different amino acids in different species. Cell Biochem Funct. 31 (4), 298-304 (2013).
- Zhang, Y. W., et al. Enhanced growth of human met-expressing xenografts in a new strain of immunocompromised mice transgenic for human hepatocyte growth factor/scatter factor. Oncogene. 24 (1), 101-106 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved