A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Methionine Functionalized Biocompatible Block Copolymers for Targeted Plasmid DNA Delivery
* These authors contributed equally
In This Article
Summary
This work presents the preparation of methionine functionalized biocompatible block copolymers (mBG) via the reversible addition-fragmentation chain transfer (RAFT) method. The plasmid DNA complexing ability of the obtained mBG and their transfection efficiency were also investigated. The RAFT method is very beneficial for polymerizing monomers containing special functional groups.
Abstract
Reversible addition-fragmentation chain transfer (RAFT) polymerization integrates the advantages of radical polymerization and living polymerization. This work presents the preparation of methionine functionalized biocompatible block copolymers via RAFT polymerization. Firstly, N,N-bis(2-hydroxyethyl)methacrylamide-b-N-(3-aminopropyl)methacrylamide (BNHEMA-b-APMA, BA) was synthesized via RAFT polymerization using 4,4’-azobis(4-cyanovaleric acid) (ACVA) as an initiating agent and 4-cyanopentanoic acid dithiobenzoate (CTP) as the chain transfer agent. Subsequently, N,N-bis(2-hydroxyethyl)methacrylamide-b-N-(3-guanidinopropyl)methacrylamide (methionine grafted BNHEMA-b-GPMA, mBG) was prepared by modifying amine groups in APMA with methionine and guanidine groups. Three kinds of block polymers, mBG1, mBG2, and mBG3, were synthesized for comparison. A ninhydrin reaction was used to quantify the APMA content; mBG1, mBG2, and mBG3 had 21%, 37%, and 52% of APMA, respectively. Gel permeation chromatography (GPC) results showed that BA copolymers possess molecular weights of 16,200 (BA1), 20,900(BA2), and 27,200(BA3) g/mol. The plasmid DNA (pDNA) complexing ability of the obtained block copolymer gene carriers was also investigated. The charge ratios (N/P) were 8, 16, and 4 when pDNA was complexed completely with mBG1, mBG2, mBG3, respectively. When the N/P ratio of mBG/pDNA polyplexes was higher than 1, the Zeta potential of mBG was positive. At an N/P ratio between 16 and 32, the average particle size of mBG/pDNA polyplexes was between 100-200 nm. Overall, this work illustrates a simple and convenient protocol for the block copolymer carrier synthesis.
Introduction
In recent years, gene therapy has emerged for the therapeutic delivery of nucleic acids as drugs to treat all kinds of diseases1. The development of gene drugs including plasmid DNA (pDNA) and small interfering RNA (siRNA) relies on the stability and efficiency of the drug delivery system (DDS)2. Among all DDS, cationic polymer carriers have the advantages of good stability, low immunogenicity, and facile preparation and modification, which give cationic polymer carriers broad application prospects3,4. For practical applications in biomedicine, researchers must find a cationic polymer carrier with high efficiency, low toxicity, and good targeting ability5. Among all polymer carriers, block copolymers are one of the most widely used drug delivery systems. Block copolymers are intensively studied for their self-assembly property and abilities to form micelles, microspheres, and nanoparticles in drug delivery5. Block copolymers can be synthesized via living polymerization or click chemistry methods.
In 1956, Szwarc et al. raised the topic of living polymerization, defining it as a reaction without chain-breaking reactions6,7. Since then, multiple techniques had been developed to synthesize polymers using this method; thus, living polymerization is viewed as a milestone of polymer science8. Living polymerization can be classified into living anionic polymerization, living cationic polymerization, and reversible deactivation radical polymerization (RDRP)9. Living anionic/cationic polymerizations have a limited scope of application due to their strict reaction conditions10. Controlled/living radical polymerization (CRP) has mild reaction conditions, convenient disposition, and good yield and has thus been a major research focus in recent years11. In CRP, active propagation chains are reversibly passivated into dormant ones to reduce the concentration of free radicals and avoid the bimolecular reaction of propagating chain radicals. The addition polymerization can continue only if the inactive dormant propagating chains are reversibly animated into chain radicals. As one of the most promising forms of living radical polymerization, reversible addition-fragmentation chain transfer (RAFT) polymerization is a method applicable to yield block polymers with controlled molecular weight and structure, narrow molecular weight distribution, and carrying functional groups12. The key to successful RAFT polymerization is the effect of chain transfer agents, usually dithioesters, which possess very high chain transfer constant.
In this paper, a RAFT polymerization method was designed to prepare BNHEMA-b-APMA block polymer, taking 4,4’-azobis(4-cyanovaleric acid) (ACVA) as an initiating agent and 4-cyanopentanoic acid dithiobenzoate (CTP) as a chain transfer agent. RAFT polymerization was used twice to introduce BNHEMA into the cationic polymer carriers. Subsequently, the amine groups in the APMA chain were modified with methionine and the guanidinylation reagent 1-amidinopyrazole hydrochloride. Making the use of the positive charges of the guanidinylation reagent and methacrylamide polymer skeleton structure, the cellular uptake efficiency of the obtained block polymer carriers was improved.
Protocol
1. Synthesis of BNHEMA polymer (PBNHEMA)
- Dissolve 1.87 g of N, N-bis(2-hydroxyethyl)methacrylamide (BNHEMA) in 1 mL of distilled water in a polymerization bottle.
NOTE: The polymerization bottle is a round-bottom flask with a rubber stopper and a magnetic stirrer. - Dissolve 0.03 g of 4-cyanopentanoic acid dithiobenzoate (CTP) and 0.02 g of 4,4’-azobis (4-cyanovalericacid ) (ACVA) in 0.5 mL of 1,4-dioxane in a 5 mL beaker. Then, add the CTP and ACVA solution to the polymerization bottle from step 1.1.
- Ventilate the reaction system in the polymerization bottle with nitrogen via three freeze-pump-thaw cycles.
- In detail, freeze the solution in the polymerization bottle using a condensate trap, fix the polymerization bottle to the iron support, and vacuumize and inject nitrogen into the reaction mixture via a conduit tipped with a needle (#9 needle, inner diameter 0.65 mm, outer diameter 0.9 mm). Seal the polymerization bottle and thaw the solution at room temperature for 30 min.
- Repeat the freeze-pump-thaw cycles three times.
- Put the polymerization bottle into a 70 °C oil-bath and let the solution react for 24 h under the nitrogen atmosphere.
- Chill the polymerization bottle at 0 °C and open the rubber stopper to terminate the polymerization process.
- Precool acetone in a -20 °C fridge for 2 h and then mix it with the reaction solution of step 1.5 at 50:1 (v/v). After that, centrifuge at 8,200 x g for 10 min to remove acetone and collect the precipitate.
- To purify the synthesized PBNHEMA, dissolve the collected precipitate in 2 mL of pure water and then mix it with 100 mL of precooled acetone, at the ratio of 1:50 (v/v). Centrifuge the solution at 8,200 x g for 10 min and collect the precipitate. Repeat this process three times.
- Dry the produced PBNHEMA using a 50 °C vacuum drier. Once dried, weigh the powder with a balance. Calculate the yield rate according to Equation 1.
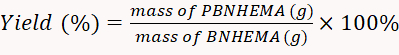 (1)
(1)
NOTE: In this experiment, the yield obtained was 77.2%.
2. Synthesis of BNHEMA-b-APMA polymer (BA)
- Dissolve 0.96 g of N-(3-aminopropyl)methacrylamide hydrochloride (APMA) and 0.93 g PBNHEMA in 5 mL of distilled water in a 10 mL beaker.
- Dissolve 0.01 g of 4,4’-azobis (4-cyanovaleric acid) (ACVA) in 0.5 mL of 1,4-dioxane and mix with the APMA-PBNHEMA solution from part 2.1.
- Transfer the mixture into a polymerization bottle and ventilate with dry nitrogen for 1 h.
- Put the polymerization bottle into a 70 °C oil-bath and let it react for 24 h under the nitrogen atmosphere.
- Chill the polymerization bottle at 0 °C and open the rubber stopper to terminate the polymerization process.
- Transfer the solution to the chilled acetone from step 1.6, and then centrifuge the solution at 8,200 x g for 10 min to precipitate the BA.
- Dissolve the BA in 2 mL of distilled water and precipitate the polymer in chilled acetone. Repeat three times.
- Dry the produced BA in a 50 °C vacuum drier and weigh the obtained powder. Calculate the yield rate according to Equation 2.
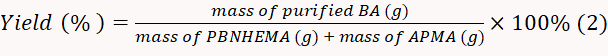
NOTE: In this experiment, the yield rate was calculated to be 82.0%.
3. Determine the mole percent of APMA in BA copolymer via the ninhydrin method
NOTE: Spectrophotometry is used to determine the contents of multicomponent amino acids. The principle is a color reaction of ninhydrin and amino acid where the absorbance is correlated with the amino acid content to a certain extent13,14.
- Dissolve 5 g of ninhydrin in 125 mL of boiling distilled water. Also, dissolve 5 g of Vitamin C in 250 mL of warm distilled water. Add the 250 mL of Vitamin C solution dropwise to the ninhydrin solution under magnetic stirring. Continue to stir for 15 min and then chill the reaction solution in a 4 °C fridge.
- Take the solution out of the fridge and filter by suction using a Buchner funnel to obtain reduced ninhydrin. Collect the precipitate and preserve it in a phosphorus pentoxide dehydrator.
- Dissolve 85 mg of ninhydrin and 15 mg of reduced ninhydrin in 10 mL of ethylene glycol monomethyl ether to prepare the ninhydrin-coloring solution.
NOTE: Ninhydrin-coloring solution can react with α-amino in the APMA and form a violet compound with a structure as described in a previous study15. - Dilute 1 mL of 0, 1, 10, 100, 1,000 mg/mL APMA monomer solutions with 1 mL of acetate buffer (2 M, pH 5.4), and then add 1 mL of ninhydrin-coloring solution, respectively.
- Heat the mixtures for 15 min in a boiling water bath and then cool them using running water. Let the solutions sit for 5-10 min and dilute them with 3 mL of 60% ethyl alcohol and mix them thoroughly. Measure the absorbance at 570 nm using a spectrophotometer and draw the standard curve (Equation 3).
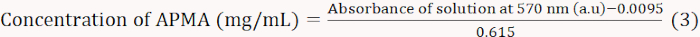
NOTE: Equation 3 was derived from the linear fitting of the absorbance at 570 nm versus the APMA concentration. - Dissolve 0.01 g of BA in 1 mL of distilled water; add 1 mL of acetate buffer (2 M, pH 5.4) and 1 mL of ninhydrin-coloring solution. Calculate the molar content of APMA according to the absorbance at 570 nm.

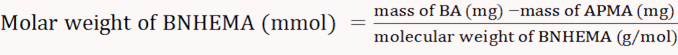 (5)
(5)

NOTE: The calculation formulas are as follows (Equations 3-6).
4. Synthesis of methionine grafted BA polymer (mBA)
- Dissolve 8.9 mg of Fomc-Methionine in 5 mL of DMSO in a recovery flask.
- Add 6.92 mg of 1-ethyl-3-(3-dimethylaminopropyl ) carbodiimide hydrochloride (EDCl) and 4.86 mg of 1-hydroxybenzotriazole (HOBT) to the recovery flask and react at 0 °C for 0.5 h.
- Dissolve 2.59 g of BA in 5 mL of DMSO solution and then add 50 μL of trimethylamine; add this solution dropwise to the recovery flask (step 4.2) and let the solution react for 0.5 h at room temperature.
- Dialyze to remove DMSO and trimethylamine from the BA solution in step 4.3 using a dialysis bag (MWCO 10 kDa) in a 2 L beaker for 24 h; replace the deionized water every 6 h.
- Freeze-dry the obtained mBA and weigh to calculate the yield rate according to Equation 7.
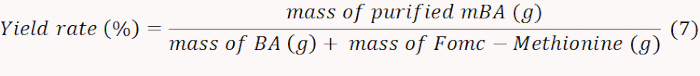
NOTE: In this case, the yield rate was determined to be 71%. - Quantify the NH2 containing mBA by measuring the absorbance at 570 nm to calculate the amount of grafted methionine. Calculate the molar content of methionine according to Equation 8.

5. Synthesis of guanidinated and methionine conjugated BNHEMA-b-APMA polymer (mBG)
NOTE: Three different mBA1, mBA2 and mBA3 copolymer were synthesized. mBA3 copolymer is used as an example in the following steps.
- Dissolve mBA3 containing 60 μmol of amino group in 5 mL of pure water.
NOTE: Amino group content was quantified using the ninhydrin method as described in step 3.5. - Dissolve 40.6 mg (300 μmol) of guanidinylation reagent 1-amidinopyrazole hydrochloride in mBA solutions.
- Adjust the pH to 9.0 with the saturated solution of sodium carbonate and let it stabilize for 24 h at room temperature.
- Dialyze the mBG product with deionized water using a dialysis bag in a beaker (MWCO 10 kDa, 2 L) and preserve it in the form of a freeze-dried powder.
The yield percentage was calculated to be 85% via Equation 8.
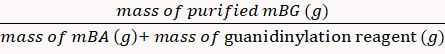 (8)
(8) - Dissolve mBG powder in D2O in the NMR tubes and characterize it using 1H nuclear magnetic resonance spectroscopy (1H NMR)16.
6. Preparation and characterization of mBG/pDNA polyplexes
- Dissolve 50 μg of pDNA in 50 μL of RNase/DNase-free water.
- Dissolve 1 mg of mBG copolymers in 1 mL of RNase/DNase-free water.
- Add the mBG copolymers solution directly into the pDNA solution according to different feeding ratios, that is, different N/P ratios (1:1, 4:1, 8:1, 16:1, and 32:1).
NOTE: N/P ratio is defined as the molar ratio of the guanidine group in the polymer and the phosphate group in pDNA, namely the molar ratio of the GPMA chain in the polymer and the mononucleotide in pDNA. The N/P ratio is calculated according to the molecular weights of amino nitrogen (N) in mBG and phosphate group (P) in pDNA . - Mix the solutions with a vortex mixer and allow them to stand for 30 min at room temperature. After that, disperse the mixture in phosphate buffer solution (PBS, pH 7.4) and preserve the obtained mBG/pDNA polyplexes at 4 °C for the follow-up experiments.
NOTE: The average particle size and Zeta potential of mBG and the complexes were detected using dynamic light scattering (DLS)17. - Dilute 10 μL of the mBG/pDNA polyplex solutions with 1 mL of PBS (pH 7.4) in the DLS and Zeta potential sample cells.
NOTE: Particle size and Zeta potential detection were performed three times and an average of the three values was taken.
7. Electrophoretic retardation experiment of mBG/pDNA polyplexes
NOTE: An electrophoretic retardation experiment was conducted to determine the minimum charge ratio.
- Take five groups of the mBG/pDNA polyplexes with different N/P ratios (1:1, 4:1, 8:1, 16:1, and 32:1) containing 50 μg of pDNA .
- Add 6x loading buffer to the mBG/pDNA polyplex samples to a final concentration of 1x.
- Add the solutions to the 1.5% agarose gels and run the gel at 90 mV for 15 min, using pDNA as control .
- Take pictures of the gels using a gel imager.
8. Cytotoxicity of mBG/pDNA polyplexes
- Seed MCF-7 cells into the 96-well plates at a density of 104 cells per well. Then, culture the cells for 12 h using DMEM medium (10% FBS and 1% antibiotic) in a humidified 37 °C incubator supplied with 5% CO2.
- Replace the culture medium with antibiotic-free DMEM culture media containing 10% fetal bovine serum (FBS) and mBG/pDNA polyplexes of different charge ratios (N/P 4, 8, 16, and 32, n=6) for 6 h, taking cells added with equal volumes of PBS solution as the control. Then, replace the culture medium with 150 μL of fresh 1640 medium and further culture the cells for 24 h.
- Add 5 mg/mL 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (20 μL/well) to the 96-well plates and further culture the cells for 4 h.
- Remove the solution and add 150 μL of DMSO to each well and shake the 96-well plates 30 sec.
- Measure the optical density (OD) at 490 nm with a microplate reader to show the cell viability. Calculate the cell viability percentage according to Equation 9.
Cell viability % = (9)
(9)
9. Transfection efficiency of mBG/ GFP-pDNA polyplexes
- Dissolve 50 μg of pDNA containing the reporter gene green fluorescent protein (GFP) pDNA (GFP-pDNA) in 50 μL of RNase/DNase-free water. Then dissolve 1 mg of mBG copolymers in 1 mL of RNase/DNase-free water. Mix pDNA and mBG solution at a charge ratio (N/P) of 1:1, 4:1, 8:1, 16:1, and 32:1 and incubate for 30 min at room temperature. Disperse the mBG/GFP-pDNA polyplexes solution using ultrasonic waves (30 s) and store at 4 °C for the follow-up experiments.
NOTE: The N/P ratio is calculated according to the molecular weights of amino nitrogen (N) in mBG and phosphate group (P) in pDNA . - Seed MCF-7 cells at a density of 2 × 105 cells per well in a 6-well plate and culture them at 37 °C and 5% CO2 in a humidified incubator for 12 h.
- Replace the culture medium with the fresh culture medium containing mBG/GFP-pDNA polyplexes of different N/P ratios (4, 8, 16, and 32) for 6 h.
- Replace the medium with 2 mL of fresh RPMI1640 medium and culture for 48 h.
- Collect the cells and detect the green fluorescence with a flow cytometer.
Results
BNHEMA was fed according to the objective degree of polymerization shown in Table 1; the synthesis procedure of mBG is shown in Figure 1. Firstly, BNHEMA homopolymer was prepared via the reversible addition-fragmentation chain transfer (RAFT) method in the water-dioxane system, using 4-cyanopentanoic acid dithiobenzoate as a chain transfer agent. Secondly, PBNHEMA was used as a chain transfer agent to prepare BNHEMA-b-APMA block polymer. APMA monomer was fed accordi...
Discussion
This study introduced a series of BNHEMA-b-APMA block polymer cationic gene carriers. These block polymers were synthesized via the reversible addition-fragmentation chain transfer (RAFT) method. The hydrophilic segment BNHEMA was introduced to improve solubility. Methionine and guanidine groups were modified to improve the target ability and transfection efficiency5. The APMA chain content increased and guanidinylation in mBG copolymer reduced the particle size of mBG/pDNA polyplexes. The particl...
Disclosures
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in this article.
Acknowledgements
This research was supported by the National Key Research and Development Program of China (No. 2016YFC0905900), National Natural Science Foundation of China (Nos. 81801827, 81872365), Basic Research Program of Jiangsu Province (Natural Science Foundation, No. BK20181086), and Jiangsu Cancer Hospital Scientific Research Fund (No. ZK201605).
Materials
| Name | Company | Catalog Number | Comments |
| 1-hydroxybenzotriazole | Macklin Biochemical Co., Ltd,China | H810970 | ≥97.0% |
| 1,4-dioxane | Sinopharm chemical reagent Co., Ltd, China | 10008918 | AR |
| 1-amidinopyrazole Hydrochloride | Aladdin Co., Ltd., China | A107935 | 98% |
| 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride | Aladdin Co., Ltd., China | E106172 | AR |
| 4,4’-azobis(4-cyanovaleric acid) | Aladdin Co., Ltd., China | A106307 | Analytical reagent (AR) |
| 4-cyano-4-(phenylcarbonothioylthio)pentanoic Acid | Aladdin Co., Ltd., China | C132316 | >97%(HPLC) |
| Acetate | Sinopharm chemical reagent Co., Ltd, China | 81014818 | AR |
| Acetone | Sinopharm chemical reagent Co., Ltd, China | 10000418 | AR |
| Agarose | Aladdin Co., Ltd., China | A118881 | High resolution |
| Ascorbic acid | Aladdin Co., Ltd., China | A103533 | AR |
| DMSO | Aladdin Co., Ltd., China | D103272 | AR |
| Ethylene glycol | Aladdin Co., Ltd., China | E103319 | AR |
| N-(3-aminopropyl)methacrylamide hydrochloride | Aladdin Co., Ltd., China | N129096 | ≥98.0%(HPLC) |
| N,N-bis(2-hydroxyethyl)methacrylamide | ZaiQi Bio-Tech Co.,Ltd, China | CF259748 | ≥98.0%(HPLC) |
| Ninhydrin | Aladdin Co., Ltd., China | N105629 | AR |
| PBS buffer | Aladdin Co., Ltd., China | P196986 | pH 7.4 |
| Plasmid DNA | BIOGOT Co., Ltd, China | pDNA-EGFP | pDNA-EGFP |
| Plasmid DNA | BIOGOT Co., Ltd, China | Pdna | pDNA |
| Sodium carbonate decahydrate | Aladdin Co., Ltd., China | S112589 | AR |
| Trimethylamine | Aladdin Co., Ltd., China | T103285 | AR |
References
- Flotte, T. R. Gene and Cell Therapy in 2018: A Look Ahead. Human Gene Therapy. 29, 1-1 (2018).
- Huang, W., et al. Nanomedicine-based combination anticancer therapy between nucleic acids and small-molecular drugs. Advanced Drug Delivery Reviews. 115, 82-97 (2017).
- Wu, Y., et al. Reversing of multidrug resistance breast cancer by co-delivery of P-gp siRNA and doxorubicin via folic acid-modified core-shell nanomicelles. Colloids & Surfaces B Biointerfaces. 138, 60-69 (2016).
- Quader, S., Kataoka, K. Nanomaterial-Enabled Cancer Therapy. Molecular Therapy. 25, 1501-1513 (2017).
- Wu, Y., et al. Multivalent methionine-functionalized biocompatible block copolymers for targeted siRNA delivery and subsequent reversal effect on adriamycin resistance in human breast cancer cell line MCF-7/ADR. Journal of Gene Medicine. 19, e2969 (2017).
- Szwarc, M. ‘Living’ Polymers. Nature. 178, 168-169 (1956).
- Szwarc, M., Rembaum, A. Polymerization of methyl methacrylate initiated by an electron transfer to the monomer. Journal of Polymer Science. 22 (100), 189-191 (1956).
- Mukhopadhyay, R. D., Ajayaghosh, A. Living supramolecular polymerization. Science. 349, 241 (2015).
- Ozkose, U. U., Altinkok, C., Yilmaz, O., Alpturk, O., Tasdelen, M. A. In-situ preparation of poly(2-ethyl-2-oxazoline)/clay nanocomposites via living cationic ring-opening polymerization. European Polymer Journal. 88, 586-593 (2017).
- Wu, W., Wang, W., Li, J. Star polymers: Advances in biomedical applications. Progress in Polymer Science. 46, 55-85 (2015).
- Boyer, C., et al. Copper-Mediated Living Radical Polymerization (Atom Transfer Radical Polymerization and Copper(0) Mediated Polymerization): From Fundamentals to Bioapplications. Chemical Reviews. 116, 1803-1949 (2016).
- Keddie, D. J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chemical Society Reviews. 43, 496-505 (2014).
- Wu, Y., et al. Guanidinylated 3-gluconamidopropyl methacrylamide-s-3-aminopropyl methacrylamide copolymer as siRNA carriers for inhibiting human telomerase reverse transcriptase expression. Drug Delivery. 20, 296-305 (2013).
- Qin, Z., Liu, W., Guo, L., Li, X. Studies on Guanidinated N-3-Aminopropyl Methacrylamide-N-2-Hydroxypropyl Methacrylamide Co-polymers as Gene Delivery Carrier. Journal of Biomaterials Science, Polymer Edition. 23, 1-4 (2012).
- Friedman, M. Applications of the Ninhydrin Reaction for Analysis of Amino Acids, Peptides, and Proteins to Agricultural and Biomedical Sciences. Journal of Agricultural and Food Chemistry. 52, 385-406 (2004).
- Habuchi, S., Yamamoto, T., Tezuka, Y. Synthesis of Cyclic Polymers and Characterization of Their Diffusive Motion in the Melt State at the Single Molecule Level. Journal of Visualized Experiments. (115), 1-9 (2016).
- Rao, D. A., Nguyen, D. X., Mishra, G. P., Doddapaneni, B. S., Alani, A. W. Preparation and Characterization of Individual and Multi-drug Loaded Physically Entrapped Polymeric Micelles. Journal of Visualized Experiments. 102, 1-5 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved