A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Purification and In Vitro Activity Assay for a (p)ppGpp Synthetase from Clostridium difficile
In This Article
Summary
Here, we describe a method for purifying histidine-tagged pyrophosphokinase enzymes and utilizing thin layer chromatography of radiolabelled substrates and products to assay for the enzymatic activity in vitro. The enzyme activity assay is broadly applicable to any kinase, nucleotide cyclase, or phosphor-transfer reaction whose mechanism includes nucleotide triphosphate hydrolysis.
Abstract
Kinase and pyrophosphokinase enzymes transfer the gamma phosphate or the beta-gamma pyrophosphate moiety from nucleotide triphosphate precursors to substrates to create phosphorylated products. The use of γ-32-P labeled NTP precursors allows simultaneous monitoring of substrate utilization and product formation by radiography. Thin layer chromatography (TLC) on cellulose plates allows rapid separation and sensitive quantification of substrate and product. We present a method for utilizing the thin-layer chromatography to assay the pyrophosphokinase activity of a purified (p)ppGpp synthetase. This method has previously been used to characterize the activity of cyclic nucleotide and dinucleotide synthetases and is broadly suitable for characterizing the activity of any enzyme that hydrolyzes a nucleotide triphosphate bond or transfers a terminal phosphate from a phosphate donor to another molecule.
Introduction
Kinase and pyrophosphokinase (or diphospho-kinase) enzymes transfer phosphates from nucleotide triphosphate (NTP) precursors to substrate molecules. The substrates can include other nucleotides, amino acids or proteins, carbohydrates, and lipids1. Bioinformatic analyses can sometimes predict an enzyme's cognate substrate or substrates based on the similarity to characterized enzymes, but experimental validation is still necessary. Similarly, the affinity of an enzyme for its substrate(s) and the rate at which it catalyzes the phosphor-transfer reaction, and the effects of co-factors, inhibitors, or other enzyme effectors must be determined experimentally. To avoid depletion of the ATP precursor by other ATP-consuming enzymes present in bacterial cytoplasm, quantitative activity assays require purified protein.
Protein purification by metal affinity chromatography has been covered thoroughly in the literature2,3. Histidine tags consisting of six consecutive histidine residues appended to the N- or C-terminus of a recombinant protein allow rapid purification by metal affinity chromatography4,5,6. These sequences are small compared to the proteins they modify and typically have a minimal effect on protein function, although they can sometimes alter protein stability and/or enzyme kinetics7,8. Histidine tags at the N- and C-termini of the same protein can have different effects, which are difficult to predict without knowing the structure of the protein in question. Histidine tags are typically incorporated during the cloning of a recombinant protein by designing primers that encode six histidine residues, either immediately 3' to the ATG start codon or immediately 5' to the stop codon of the open reading frame. After amplification, the hexahistidine-containing gene is ligated into a vector under the control of an inducible promoter and expressed, typically in a laboratory strain of E. coli. The recombination protein can then be isolated on an affinity resin containing immobilized divalent cations (typically nickel or cobalt)9. Contaminating native metal-binding proteins can be removed by titration with imidazole, which competitively displaces bound protein2. Finally, the target protein is eluted from the column with higher concentrations of imidazole. There are several commercial sources for immobilized metal cation resins, and the manufacturers provide recommendations for the buffer conditions and imidazole concentrations. After elution, protein may be analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), dialyzed, or used immediately in functional assays.
There are several methods to indirectly monitor kinase activity by coupling ATP phosphate bond hydrolysis to a second reaction that releases or excites a fluorophore or generates chemiluminescence, but these reactions have multiple moving parts and can be logistically challenging10. The most straightforward way to specifically measure phosphor-transfer activity is to directly monitor the transfer of a radiolabeled phosphate group from a commercially available γ-32-P NTP precursor to a non-radiolabeled substrate11,12,13. Mixtures of radiolabeled substrates and products can be separated and quantified by thin layer chromatography (TLC). TLC utilizes the differential mobility of solutes in a given solvent by allowing the solvent (liquid phase) to migrate by capillary action across a surface (solid phase) upon which a mixture of solutes has been adsorbed14. Solutes that are small and/or lack favorable interactions with the solid phase will migrate longer distances from their initial location than solutes with higher molecular weights or great affinities for the solid. For examination of phosphor-transfer, phosphate moieties increase the molecular weight of molecules they are added to, and add negative ionic charge at neutral or acidic pH11,12,14. This decreases their mobility on a basic surface such as PEI-cellulose. When developed in acidic potassium phosphate buffer, mixtures of mono-, di-, tri-, tetra-, and pentaphosphate species can be readily separated on PEI-cellulose, allowing quantification of each species (Figure 2, Figure 3). Such assays can be performed using cell lysates containing the enzyme of interest, but this includes the potential for the activity of other kinases, phosphatases, and general ATPases to deplete the substrate and/or product. For a quantitative in vitro assessment of enzyme activity, it is necessary to purify the enzyme of interest.
Guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) are ribonucleotide signaling molecules formed by the transfer of a pyrophosphate group from an adenosine triphosphate (ATP) precursor to, respectively, a guanosine diphosphate (GDP) or guanosine tetraphosphate (GTP) substrate15. These single ribonucleotide signals, collectively known as (p)ppGpp, mediate a cell-wide response to environmental stress known as the stringent response in diverse bacterial species15,16. Two conserved classes of enzymes catalyze the formation of (p)ppGpp15,17 Rel/Spo homolog (RSH) enzymes are 'long' bifunctional (p)ppGpp synthetase/hydrolases named for their similarity to the RelA and SpoT (p)ppGpp metabolic enzymes from Escherichia coli which contain synthetase, hydrolase, and regulatory domains, while small alarmone synthetase (SAS) enzymes are short monofunctional synthetases found exclusively in Gram positive bacteria15,17,18. The spore-forming Gram-positive bacterium Clostridium difficile encodes putative RSH and SAS genes19. Here, we present initial activity assays that confirm that the C. difficile RSH enzyme is a catalytically active (p)ppGpp synthetase.
Protocol
1. Inducible Overexpression of a Histidine-tagged Protein
- Amplify rsh from C. difficile R20291 genomic DNA.
- Use a high-fidelity polymerase and follow the manufacturer’s instructions.
- Amplify C. difficile rsh using primers
rsh_F(CAGGTACCGGTTATATGCATGATAAAGAATTACAAG) and
rsh_R(CCCTGCAGCTAATGGTGATGGTGATGGTGATTTGTCATTCTATAAATAC), which introduces a C-terminal hexahistidine tag.
NOTE: The KpnI and PstI cut sites in the primer sequences are bolded. - Digest the pMMBneo vector and the rsh-his6 PCR product with Pstl and KpnI restriction cut sites at 37 °C for 45 min.
- Purify the linearized vector and the PCR fragments via agarose gel electrophoresis and subsequent purification with a DNA gel extraction kit.
- Measure the 280 nm absorbance (A280) of the vector and the amplified PCR product. Use the equation
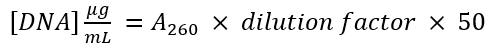 to determine the DNA concentration of each DNA fragment.
to determine the DNA concentration of each DNA fragment.
- Ligate rsh into pMMBneo for expression.
- Combine 25 ng of digested PMMBneo vector, 125 ng of rsh-his6 gene product, 2 μL of 10x ligase buffer, and 1 μL of DNA ligase. Use the nuclease free water to adjust the total volume to 20 μL. Incubate at 16 °C for 16 h.
NOTE: The efficacy of ligation depends on fragment size and must be adjusted based on manufacturer protocols. - Transform the ligated vector product into E. coli DH5-α or another recA- plasmid maintenance strain. Select for transformed cells on LB plates with 100 μg/mL kanamycin and incubate for 16–24 h at 37 °C.
- Pick four colonies from the transformation plate (s) and streak each on a fresh plate containing 100 μg/mL kanamycin for DNA isolation. Incubate the new plates at 37 °C for 16–24 h and select one isolate for the subsequent protein expression.
- To confirm the successful ligation of the intact rsh protein coding region, pick a colony of the chosen isolate into 20 μL of water, heat at 95 °C for 10 min, and use as a template for a confirmatory PCR using the same primer used to amplify the gene.
- Combine 25 ng of digested PMMBneo vector, 125 ng of rsh-his6 gene product, 2 μL of 10x ligase buffer, and 1 μL of DNA ligase. Use the nuclease free water to adjust the total volume to 20 μL. Incubate at 16 °C for 16 h.
- Transform the verified plasmid according to standard transformation protocols for E. coli bacterial plasmid transformation into E. coli BL21 system for high yield protein production.
- Express RSH-His6 in E. coli.
- Select a single colony of E. coli BL21 transformed with pMMBneo::rsh and inoculate 2 mL of LB medium with 50 μg/mL kanamycin. Incubate at 37 °C 12–16 h while shaking at 250 rpm.
- Inoculate 500 mL of LB medium containing 50 μg/mL kanamycin with 0.5 mL of the overnight culture for cell growth and protein expression.
- Incubate the expression culture at 37 °C and 250 rpm in an incubator shaker until the cell density reaches an OD600 0.167 at 37 °C.
- Reduce the incubator temperature to 30 °C and wait 30 min for the culture temperature to drop.
- Induce RSH expression by adding isopropyl β-D-thiogalactoside (IPTG) to a final concentration of 0.5 mM. Allow the induction to take place overnight (for 16–18 h) at 30 °C, shaking at 250 rpm.
- Pellet by centrifugation at 3,080 x g for 30 min at 4 °C.
NOTE: The pellet can be stored overnight at -20 °C for purifying target protein the following day without noticeable loss of protein yield or enzymatic activity.
2. Protein Purification by Nickel Affinity Chromatography
NOTE: Continue directly with protein purification steps provided below after clarifying the cell lysate. Storing clarified lysate at 4 °C overnight for subsequent protein purification reduces the protein yield.
- Purify protein using 1 mL of Nickel-Nitriloacetic Acid (Ni-NTA) resin on a gravity column.
- The day before use, equilibrate the column overnight at 4 °C with 2 mL of equilibration buffer (10 mM Tris-HCl pH 7.79, 300 mM NaCl, 50 mM NaH2PO4, 0.5 mg/mL lysozyme, 5 mM MgCl2, 10 mM imidazole, 0.25 mM DTT, 5 mM phenylmethane sulfonyl fluoride (PMSF), 10% glycerol).
- The following day bring the column from 4 °C to RT prior to loading the clarified lysate and let it stand for ~2–3 h.
NOTE: Bringing the column to thermal equilibrium is crucial to avoid air bubbles forming within the column. - Resuspend the pellet obtained in step 1.3.6 in the lysis buffer (10 mM Tris-HCl pH 7.8, 300 mM NaCl, 5 mM MgCl2, 50 mM NaH2PO4, 10% glycerol, 0.5 mg/mL lysozyme, 10 mM imidazole, 0.25 mM DTT and 5 mM PMSF).
- Sonicate cells on ice for 8 x 10 s intervals, pausing 30 s between pulses.
- Clarify the lysate by centrifugation at 3,080 x g for 30 min at 4 °C using a microcentrifuge.
- Prepare lysate with equal volumes of lysis buffer then apply the prepped clarified lysate to the column and collect the flow-through.
- Reapply clarified lysate flow-through to the column and collect the secondary flow-through.
- Wash the column with wash buffer 1 (10 mM Tris-HCl (pH 7.79), 300 mM NaCl, 5 mM MgCl2, 50 mM NaH2PO4, 30 mM imidazole, 10% glycerol). Collect the flow-through.
NOTE: Inclusion of 5 mM MgCl2 in the wash and elution buffers is important for enzymatic activity of the purified protein. - Wash the column with wash buffer 2 (10 mM Tris-HCl (pH 7.79), 300 mM NaCl, 5 mM MgCl2, 50 mM NaH2PO4 and 50 mM imidazole). Collect the flow-through.
- Apply 2 mL elution buffer (10 mM Tris-HCl (pH 7.79), 300 mM NaCl, 50 mM NaH2PO4, 10% glycerol and 75 mM imidazole). Collect flow-through in two fractions of 1 mL each.
NOTE: The column after this step can be stored in equilibration buffer at 4 °C if the same protein will be purified in the next purification assay. The column can be used up to 3 times if stored properly.
- Assess column fractions for purity by SDS-PAGE.
- To qualitatively assess protein purification, run 20 μL aliquots of all column fractions on a 4%/10% polyacrylamide gel for 60 min at 170 V.
- Stain the gel with 0.1% Coomassie blue at room temperature for 5 h, rocking gently on a benchtop rocker.
- Destain the gel in 40% methanol, 10% glacial acetic acid overnight at room temperature, rocking on a benchtop rocker.
NOTE: A representative gel is pictured in Figure 1.
- Dialyze elute fraction 2 overnight at 4 °C.
- Dialyze against dialysis buffer (15.7 mM Tris-HCl (pH 7.6), 471.9 mM NaCl, 15.69 mM MgCl2, 1.57 mM DTT, 1.5 mM PMSF, and 15.7% glycerol) at a 200:1 ratio using a 1 mL dialysis device with a 20 kDa molecular weight cut-off (MWCO).
- Determine the concentration of dialyzed protein sample by measuring absorbance at 280 nm and using the calculated molar extinction coefficient 82085 M-1cm-1 20.
- Store 5 μL aliquots of the dialyzed protein sample in aliquots at -80 °C until use.
3. Protein Activity Assay by Thin Layer Chromatography
- Prepare the thin layer chromatography plate.
- Prior to performing the reaction, prepare polyethyleneimine (PEI)-cellulose plates by washing in deionized water. Place the plates in a glass chamber with double distilled water to a depth of ~0.5 cm.
- Allow water to migrate to the top of the plate.
NOTE: Washing the plates is not strictly necessary, as plates may be used without it, but washing does increase the clarity of resulting images. Washing the plates in two perpendicular directions further ensures that any contaminants present in the resin are isolated in one corner of the plate (Figure 2). - Bring the plates out of the glass chamber and leave on a benchtop rack to dry overnight (12–18 h).
- Mark the dry plates 2.0 cm from one edge with a soft pencil to indicate where the samples will be applied for TLC. For 2 μL samples, apply samples no less than 1.0 cm apart (Figure 2).
NOTE: This allows clear separation between adjacent spot, which is critical for signal quantification. As long as the cellulose resin is not scratched, small pencil marks on the surface will not interfere with solvent migration. - When planning experiments, always leave one spot on each plate unused.
NOTE: This will provide a blank lane for sample quantification (Figure 2). A 20 cm TLC plate will have room for 19 spots.
- Enzyme activity assay
- Prepare a 5x buffer mix containing 50 mM Tris-HCl (pH 7.5), 25 mM ammonium acetate, 10 mM KCl, 1 mM DTT and 0.6 mM ATP.
NOTE: This mix may be prepared in large quantities and frozen in 10 μL aliquots for later use. Do not subject mix to multiple freeze-thaw cycles. - Prepare individual reactions containing 3 μM RSH, 1x buffer mix, 0.6 mM GDP, 1.2 mM MgCl2. Add 1.0 μCi of γ-32P-ATP per 10 μL of reaction and use nuclease free water to bring the reaction up to a desired volume. Add the RSH after the other components have been mixed, as the addition of RSH to the nucleotide-containing mix initiates the enzymatic activity assay.
NOTE: Final reaction volume will depend on the number of timepoints sampled. To sample 2 μL/timepoint, assemble 10 μL of reaction mixture for each 4 timepoints. - To control for ATP hydrolysis from contaminating nuclease activity, assemble a 10 μL reaction containing no protein and incubate it in parallel. Spot 2 μL samples at t = 0 and at the end of the experiment to ensure that ATP was not hydrolyzed in the absence of protein.
- Immediately upon addition of RSH, remove 2 μL and spot it onto the labeled PEI-cellulose plate as the t = 0 min sample.
- Incubate the reaction at 37 °C, removing 2 μL aliquots at desired timepoints.
NOTE: Enzymatic activity will cease when the sample is adsorbed onto the cellulose plate. Wait 10–30 min after the last spot is added to plate before development to ensure complete adsorption and sample drying.
- Prepare a 5x buffer mix containing 50 mM Tris-HCl (pH 7.5), 25 mM ammonium acetate, 10 mM KCl, 1 mM DTT and 0.6 mM ATP.
- Thin layer chromatography
- Fill the chromatography chamber with 1.5 M 1.5 M KH2PO4 (pH 3.64) to a depth of 0.5 cm.
NOTE: The volume needed will depend on the dimensions of the chromatography tank. Any glass container with a level bottom that is wide enough to allow insertion of the TLC plate without bending may be used as a developing tank with the addition of a cover. The TLC plate can be cut into narrower strips with a clean razorblade to enable development in a glass beaker covered in plastic film. - Immerse the bottom edge of the plate in solvent. Allow the solvent to migrate to the top of the plate (~90 min).
NOTE: While solvent migration will halt at the top of the plate and samples will not be lost or run together during a longer immersion, plates should not be left in solvent overnight. Immersions longer than 4 h can cause the resin to detach from the plate backing and result in loss of signal. - Remove the plate from the chromatography tank and place it on a benchtop drying rack.
- Allow the plate to air dry overnight.
NOTE: Drying may be accelerated by the use of a hair drier. Dryness can be assessed by the color of the resin, which will darken when wet and return to the color of an unused plate when completely dry. - After the plate is dry, wrap the plate in plastic film to avoid transfer of radioactive material to the imaging cassette and analyze by autoradiography (Figure 3).
- Fill the chromatography chamber with 1.5 M 1.5 M KH2PO4 (pH 3.64) to a depth of 0.5 cm.
- Data analysis
- Expose the PEI-cellulose plate containing separated reactions to a phosphorimager cassette for 4 h at room temperature.
NOTE: This is sufficient exposure to yield a very clear image using the indicated concentrations of fresh γ-32P-ATP. If lower amounts of radiolabelled substrate are used, exposure time can be increased to 12-16 h. - Image the cassette on a phosphorimager.
- Using imaging software with a graphical user interface, draw Regions of Interest (ROIs) by selecting Draw Rectangle and using the mouse to draw rectangular ROIs around one entire lane and the ATP and ppGpp spots contained within that lane (Figure 3).
- Use the Select, Copy, and Paste commands (or corresponding commands based on the imaging software being used) to draw identical ROIs within the other lanes to ensure that the ROIs are measuring signal within identical areas in each lane. Include ROIs from an unused lane, to be used as blanks.
- Using the Analyze | Tools | ROI Manager | Add commands of the imaging software, select all of the ROIs drawn on the PEI cellulose plate.
- Using the Analyze | Set Measurements | Measure commands, quantify the signal intensity within each ROI and export the measurements as a spreadsheet (Figure 3). Subtract blank ROI values from experimental signals.
- Calculate what percentage of the blanked signal within each lane is attributable to ATP and ppGpp using the formulas

NOTE: ROIs may be drawn and quantitated using commercial software compatible with the phosphorimager or freely available ImageJ software (National Institutes of Health).
- Expose the PEI-cellulose plate containing separated reactions to a phosphorimager cassette for 4 h at room temperature.
Results
We present a method for the affinity purification of a (p)ppGpp synthetase from Clostridium difficile and the assessment of its enzymatic activity. Figure 1 demonstrates the protein purification achieved by metal affinity chromatography. The second elution (E2) fraction from this purification was dialyzed and used for the enzymatic activity assay. Figure 2 details the necessary steps to prepare for and carry out pyrophos...
Discussion
Here we report the purification of His-tagged RSH from C. difficile and present a method for activity quantification using radiolabeled thin layer chromatography. This method has previously been used to assess the activity of diguanylate cyclase enzymes from C. difficile, as well as (p)ppGpp synthetase, nucleotide cyclase, kinase and phosphodiesterase enzymes from other organisms11,12,13,
Disclosures
The authors declare no competing financial interests or other conflicts of interest.
Acknowledgements
This work was funded by NIAID 1K22AI118929-01. EBP was supported by a Summer Research Fellowship Program Grant from the Office of Research at Old Dominion University, Norfolk, Virginia, USA.
Materials
| Name | Company | Catalog Number | Comments |
| Inducible overexpression of a histidine-tagged protein | |||
| Phusion polymerase | New England Biolabs (NEB) | M0530L | |
| QIAEX II DNA Gel Extraction Kit | Qiagen | 20021 | |
| KpnI restriction enzyme | NEB | R0142S | |
| PstI restriction enzyme | NEB | R0140S | |
| T4 DNA ligase | NEB | M0202 | |
| NEB® 5-alpha Competent E. coli (High Efficiency) | NEB | C2987I | |
| BL21 (DE3) Competent E. coli | NEB | C2527I | |
| IPTG | Sigma-Aldrich | 10724815001 | |
| JXN-26 centrifuge with JLA 10.500 rotor | Beckman Coulter Avanti | - | |
| Microcentrifuge with D3024/D3024R rotor | Scilogex | - | |
| MaxQ SHKE6000 Incubator | Thermo Scientific | - | |
| Ultrasonic processor | Sonics | VC-750 | |
| Protein purification by nickel affinity chromatography | |||
| Ni-NTA resin | G Biosciences | 786-940/941 | |
| Pierce Disposable Gravity columns, 10 mL | Thermo Scientific | 29924 | |
| 1 mL Spectra/ Por float-A-lyzer G2 dialysis device (MWCO: 20-kD) | Spectrum | G235033 | |
| Mini-Protean Electrophoresis Cell | BioRad | 1658004 | |
| Protein activity assay by thin layer chromatography | |||
| Thin layer chromatograph (TLC) development tank | General Glass Blowing Company | 80-3 | |
| Polyethylenimine (PEI)-cellulose plates (20 cm x 20 cm, 100 μm thickness) with polyester support | Sigma-Aldrich | Z122882-25EA | |
| ATP, [γ-32P]- 3000 Ci/mmol 10mCi/ml lead, 100 μCi | Perkin Elmer | NEG002A | |
| Adenosine 5’-triphosphate (ATP) 100 mM | Bio Basic Canada | AB0311 | |
| Guanosine-5’-diphosphate disodium salt (GDP) | Alfa Aesar | AAJ61646MC/E | |
| Storage phosphor screen | GE Healthcare Life Sciences | BAS-IP TR 2040 E Tritium Screen | |
| Storm 860 phosphorimager | GE Healthcare Life Sciences | - |
References
- Cheek, S., Ginalski, K., Zhang, H., Grishin, N. V. A comprehensive update of the sequence and structure classification of kinases. BMC Structural Biology. 5 (1), 6 (2005).
- Porath, J. Immobilized metal ion affinity chromatography. Protein Expression and Purification. 3 (4), 263-281 (1992).
- Arnau, J., Lauritzen, C., Pedersen, J. Cloning strategy, production and purification of proteins with exopeptidase-cleavable His-tags. Nature Protocols. 1 (5), 2326-2333 (2006).
- Porath, J., Carlsson, J., Olsson, I., Belfrage, G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 258 (5536), 598-599 (1975).
- Smith, M. C., Furman, T. C., Ingolia, T. D., Pidgeon, C. Chelating peptide-immobilized metal ion affinity chromatography. A new concept in affinity chromatography for recombinant proteins. Journal of Biological Chemistry. 263 (15), 7211-7215 (1988).
- Graslund, S., et al. Protein production and purification. Nature Methods. 5 (2), 135-146 (2008).
- Booth, W. T., et al. Impact of an N-terminal Polyhistidine Tag on Protein Thermal Stability. ACS Omega. 3 (1), 760-768 (2018).
- Thielges, M. C., Chung, J. K., Axup, J. Y., Fayer, M. D. Influence of histidine tag attachment on picosecond protein dynamics. Biochemistry. 50 (25), 5799-5805 (2011).
- Chaga, G. S. Twenty-five years of immobilized metal ion affinity chromatography: past, present and future. Journal of Biochemical and Biophysical Methods. 49 (1-3), 313-334 (2001).
- Ma, H., Deacon, S., Horiuchi, K. The challenge of selecting protein kinase assays for lead discovery optimization. Expert opinion on drug discovery. 3 (6), 607-621 (2008).
- Tamayo, R., Tischler, A. D., Camilli, A. The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. Journal of Biological Chemistry. 280 (39), 33324-33330 (2005).
- Purcell, E. B., McKee, R. W., McBride, S. M., Waters, C. M., Tamayo, R. Cyclic Diguanylate Inversely Regulates Motility and Aggregation in Clostridium difficile. Journal of Bacteriology. 194 (13), 3307-3316 (2012).
- Purcell, E. B., Tamayo, R. Identification and characterization of cyclic nucleotide phosphodiesterases. Methods in Molecular Biology. 1016, 235-243 (2013).
- Ross, P., et al. Control of cellulose synthesis Acetobacter xylinum. A unique guanyl oligonucleotide is the immediate activator of the cellulose synthase. Carbohydrate Research. 149 (1), 101-117 (1986).
- Potrykus, K., Cashel, M. (p)ppGpp: still magical?. Annual Review of Microbiology. 62, 35-51 (2008).
- Boutte, C. C., Crosson, S. Bacterial lifestyle shapes stringent response activation. Trends in Microbiology. 21 (4), 174-180 (2013).
- Nanamiya, H., et al. Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Molecular Microbiology. 67 (2), 291-304 (2008).
- Gaca, A. O., et al. Basal Levels of (p)ppGpp in Enterococcus faecalis: the Magic beyond the Stringent Response. mBio. 4 (5), (2013).
- Sebaihia, M., et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nature Genetics. 38 (7), 779-786 (2006).
- Gasteiger, E., et al. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Research. 31 (13), 3784-3788 (2003).
- Purcell, E. B., et al. A nutrient-regulated cyclic diguanylate phosphodiesterase controls Clostridium difficile biofilm and toxin production during stationary phase. Infection and Immunity. , (2017).
- Mechold, U., Murphy, H., Brown, L., Cashel, M. Intramolecular Regulation of the Opposing (p)ppGpp Catalytic Activities of RelSeq, the Rel/Spo Enzyme from Streptococcus equisimilis. Journal of Bacteriology. 184 (11), 2878-2888 (2002).
- Dalebroux, Z. D., Svensson, S. L., Gaynor, E. C., Swanson, M. S. ppGpp Conjures Bacterial Virulence. Microbiology and Molecular Biology Reviews. 74 (2), 171-199 (2010).
- Bartlett, J. G. Clostridium difficile: progress and challenges. Annals of the New York Academy of Sciences. 1213, 62-69 (2010).
- . . US Department of Health and Human Services. , (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved