A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Investigation of Plant Interactions Across Common Mycorrhizal Networks Using Rotated Cores
In This Article
Summary
Most plants within communities likely are interconnected by arbuscular mycorrhizal (AM) fungi, but mediation of plant interactions by them has been investigated primarily by growing plants with versus without mycorrhizas. We present a method to manipulate common mycorrhizal networks among mycorrhizal plants to investigate their consequences for plant interactions.
Abstract
Arbuscular mycorrhizal (AM) fungi influence plant mineral nutrient uptake and growth, hence, they have the potential to influence plant interactions. The power of their influence is in extraradical mycelia that spread beyond nutrient depletion zones found near roots to ultimately interconnect individuals within a common mycorrhizal network (CMN). Most experiments, however, have investigated the role of AM fungi in plant interactions by growing plants with versus without mycorrhizal fungi, a method that fails to explicitly address the role of CMNs. Here, we propose a method that manipulates CMNs to investigate their role in plant interactions. Our method uses modified containers with conical bottoms with a nylon mesh and/or hydrophobic material covering slotted openings, 15N fertilizer, and a nutrient-poor interstitial sand. CMNs are left either intact between interacting individuals, severed by rotation of containers, or prevented from forming by a solid barrier. Our findings suggest that rotating containers is sufficient to disrupt CMNs and prevent their effects on plant interactions across CMNs. Our approach is advantageous because it mimics aspects of nature, such as seedlings tapping into already established CMNs and the use of a suite of AM fungi that may provide diverse benefits. Although our experiment is limited to investigating plants at the seedling stage, plant interactions across CMNs can be detected using our approach which therefore can be applied to investigate biological questions about the functioning of CMNs in ecosystems.
Introduction
Arbuscular mycorrhizal (AM) fungi assisted plants in the colonization of land 460 million years ago1 and today, they are ubiquitous symbionts of most plants2, providing them with vital mineral nutrients for growth. The thin, thread-like hyphae of AM fungi forage for mineral nutrients beyond nutrient depletion zones near roots, often encountering and colonizing root systems of neighboring plants in a “common mycorrhizal network” (CMN). Common mycorrhizal networks also may form when fungal germlings join established networks3, or when AM hyphae fuse (anastomose) with conspecific hyphae4,5,6,7. The extent of these extraradical hyphae in the soil is enormous, with extraradical hyphae constituting 20% to 30% of total soil microbial biomass in prairie and pasture soils8 and stretching for 111 m·cm-3 in undisturbed grassland9.
Common mycorrhizal networks partition mineral nutrients among interconnected neighboring plants10,11,12,13. Plants may receive up to 80% of their phosphorus and 25% of their nitrogen requirements from AM fungi, while providing up to 20% of their total fixed carbon to the fungi in return14. Recent in vitro root organ culture work has found that CMNs preferentially exchange mineral nutrients with host roots that provide the most carbon to the fungi11,12. Furthermore, different species of AM fungi may differ in their quality as symbiotic partners, with some fungi exchanging more phosphorus for less carbon than others15. Although root organ cultures are beneficial models for studying the AM symbiosis because they present carefully controlled environments and the ability to directly observe hyphal interconnections, they do not include photosynthesizing shoots which affect important physiological processes such as photosynthesis, transpiration, and diurnal changes, as well as constituting carbon and mineral nutrient sinks.
In nature, seedlings most likely tap into already established CMNs. Until recently, however, scientists have only examined the impact of AM fungi on plant nutrition by growing plants with and without AM fungi, often with a single species of AM fungus. Although this work has been tremendously informative to our understanding of arbuscular mycorrhizas, this method has overlooked the potentially crucial role that CMNs may have in interactions among interconnected host plants. In particular, plants that are highly dependent on AM fungi for growth interact minimally without AM fungi16,17, possibly confounding our interpretation of AM fungus-mediated interactions when used as ‘controls’ for baseline reference.
We propose a rotated-core approach for investigation of the role of CMNs in plant interactions and population structuring. Our approach mimics components of the AM symbiosis in nature because whole plants join established CMNs and all plants are grown with AM fungi. By removing root interactions, our methodology specifically focuses on interactions mediated by AM fungi while also tracking mineral nutrient movement within CMNs. Our approach builds on previous work that has used rotated cores both in the field and in the greenhouse to understand AM functioning realistically.
The rotated core method has been established in the literature as a method to manipulate extraradical hyphae18,19,20,21, and it has had several reincarnations depending on its purpose over the past two decades. Initially, mesh bags or barriers allowing in-growth of hyphae were used to provide root-free compartments to quantify the amount of arbuscular mycorrhizal hyphae in the soil22,23. Then, cylindrical cores of soil enclosed in rigid water pipes or plastic tubing with slots covered in a nylon mesh penetrable by hyphae, but not roots, were developed. These could easily be rotated to disrupt extraradical mycelia18,24,25. The rotated cores were placed between plants, and soil hyphal lengths per gram of soil18, 13C fluxes to extraradical mycelia24, or phosphorus uptake from plant-free cores were quantified18. Another use of such cores was to grow plants within them in the field to reduce colonization of roots by AM fungi through frequent hyphal disruption as an alternative to sterilization or the application of fungicides, both of which have indirect effects on soil organic matter and other microbes18.
The hyphal mesh barrier approach has been used to investigate nutrient partitioning and plant interactions across CMNs, but in rectangular microcosms rather than with rotated cores. Walder et al.26 investigated interactions between Linum usitatissimum (flax) and Sorghum bicolor (sorghum) by tracing mineral nutrient for carbon exchange using isotopes across CMNs of either of the AM fungi Rhizophagus irregularis or Funneliformis mosseae26. The microcosms in their study comprised plant compartments separated by mesh barriers, hyphal compartments only accessible to mycorrhizal hyphae, and labeled hyphal compartments that contained radioactive and stable isotopes. As controls, the study used treatments without mycorrhizal fungi. Song et al.27 used a similar approach to find that plant signals could be carried only among established CMNs of F. mosseae when one plant was infected by a fungal pathogen. Also, similarly to Walder et al.26, Merrild et al.28 grew plants in individual compartments separated by mesh to investigate plant performance of Solanum lycopersicum (tomato) seedlings linked by CMNs to a large Cucumis sativus (cucumber) plant that represented an abundant carbon source. They also used treatments without mycorrhizal fungi instead of severing CMNs28. In a second, related experiment, carbon for phosphorus exchange was examined using mesh bags labeled with 32P. Microcosms with hyphal mesh barriers and CMN severing as a treatment were used by Janos et al.29, who investigated competitive interactions between seedlings of the savanna tree species Eucalyptus tetrodonta and transplants of the rain forest tree, Litsea glutinosa. In that study, Janos et al.29 lifted compartments containing seedlings a few centimeters, sliding layers of mesh against one another to break hyphal interconnections29.
The final step in the evolution of the rotated core method has been to grow plants inside cores that are within pots or microcosms20,30. Wyss30 used rotated cores to ascertain if extraradical AM mycelium could colonize Pinus elliottii seedlings when spreading from a donor or ‘nurse’ AM host plant, Tamarindus indica, and how extraradical mycelium of ectomycorrhizal fungi influences seedling performance. Large commercial tubular seedling containers (Table of Materials) within microcosms were either solid plastic (no CMNs) or slotted and covered with a hydrophobic membrane. Slotted seedling containers were either not rotated (intact CMNs) or rotated to sever established CMNs. Rotated cores with different mesh barrier sizes were used by Babikova et al.20 to investigate belowground signaling through CMNs among Vicia faba (bean) plants. In their study, a central donor plant in 30 cm diameter mesocosms was interconnected either by roots and hyphae (no barrier) or only by CMNs established through a 40 μm mesh. Central plants were severed from interactions with neighboring plants through rotation of the mesh-enclosed cores, or CMNs were prevented by a fine 0.5 μm mesh enclosing the core.
Here, we present a method that combines aspects of prior rotated-core approaches to examine the influence of CMNs on direct plant interactions combined with stable isotope tracing. Our method uses a ‘target plant’ approach, in which the central plant of interest is surrounded by neighboring plants. Plants are grown inside rotatable seedling containers that are slotted and covered with nylon silk-screen mesh, hydrophobic membrane, or are non-modified solid plastic. Common mycorrhizal networks are severed once a week or kept intact, and 15N stable isotopes trace the movement of nitrogen from neighbors’ rotated cores to the central target plant. By comparing plant size with mineral nutrient and stable isotope uptake, we assess which plants may benefit or suffer from CMNs in interactions among host plants.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Construction and assembly of rotatable cores
- Modify commercial tubular seedling containers (subsequently called ‘containers’; Table of Materials) to have 19 mm wide x 48 cm length openings.
- Using a drill-press with a 19 mm hole saw without a central, pilot twist drill, cut two holes, one above the other, in the sides of a container (2.5 cm diameter x 12.1 cm length) so that the holes are about 1 cm apart. Hold the container against a fence on the drill press and have a stop with a short dowel that will fit inside the container to help hold it in place while drilling. Use a container with flexible plastic to prevent cracking.
- Cut the remaining thin piece of plastic between the holes with scissors, a wire cutter, or tin snips (for rigid plastic use a sabre-saw) to make one elongated opening about 2 cm wide and 5 cm long.
- Repeat steps 1.1.1 – 1.1.2 on the opposite side of the container.
- Cover the slots with nylon mesh and/or hydrophobic membrane (Figure 1A).
- Cut nylon mesh with 40 μm pores into 9.5 cm x 8.5 cm pieces. Cut as many pieces as there are containers.
- Glue the nylon mesh externally onto the containers to cover both openings with some slight overlap in the fabric using high-strength, industrial hot glue.
- If the prevention of water movement is needed, such as when using water-soluble nutrients or stable isotopes, cover the nylon mesh layer with a hydrophobic membrane31,32 (Table of Materials) that allows AM fungus hyphae to pass, but only the movement of water vapor and not liquid water.
- Place the hot glue around the openings on the container and along the long edges of the nylon mesh. Roll the container onto the fabric to avoid burning fingers. Add a layer of glue along the fabric edge where the mesh edges overlap. Press the edge onto some cardboard to firmly seal it. Always roll consistently in one direction that will be the same direction of the rotation of the finished containers within pots or microcosms so that the overlapped mesh edge will not be pushed to potentially dig into the substrate.
- Once the glue has cooled, tape the top and bottom ends of the fabric to the container to prevent loose edges and ripping using a flexible tape, such as electrician's tape.
- Using the same tape as in step 1.2.5, cover the small holes on the sides of the conical end (not the hole at the tip of the bottom) of each container to prevent root growth out of the container into the rest of the pot/microcosm.
- To prevent soil loss while providing drainage, place a glass marble into the bottom of each container.
- For a control treatment that does not involve any potential for a CMN to form between plants, use solid, unmodified containers (Figure 1A).
2. Assembly of pots or microcosms to fit the conical ends of the containers
- To ensure containers stand upright in a fixed position and have proper drainage, flip a pot over so that the bottom is facing up. Cut around the bottom of the pot, leaving a small lip for support, using a sabre-saw.
- Preparation of polystyrene foam
- Cut polystyrene foam, about 36 mm thick, to the same diameter as the bottom of the pot using a bandsaw with a circle-cutting jig.
- Drill holes into the foam using a drill press and 19 mm hole saw (without a central twist drill) in the pattern in which the containers will be positioned.
- For a target plant experiment, drill a central hole with equally spaced holes for neighboring individuals surrounding it. For a pot with a 15.5 cm diameter, space six holes 12 mm apart around the circumference of an 11 cm diameter circle (Figure 1B).
- Lay out the holes hexagonally or in a square array (Figure 1C, D) for a microcosm experiment.
3. Filling of the containers and pots with soil and sand mixtures
- Select a desired soil mixture and add AM fungus field-collected or pot-cultured inoculum to the soil by uniformly mixing chopped root pieces (1 – 2 cm long) thoroughly with the soil. Mix the desired soil with an infertile silica sand or glass beads to decrease the concentration of mineral nutrients available to plants.
- Position the filled containers in the drilled foam or microcosm bottom and fill the interstitial space with an infertile substrate.
- Fill the interstitial space between containers with nutrient-poor silica sand mixture using a funnel to assist in filling small spaces. To ensure adequate drainage and mimic the texture of the soil, mix medium particle-size sand, such as 6-20 grade, with small particle-size sand, such as 30-65 grade, in a cement mixer.
4. Establishment of CMNs throughout pots/microcosms
- Plant pretreatment ‘nurse’ plants of the desired species into each container to sustain AM fungi so that they can spread among the containers and establish CMNs.
- When all containers have established seedlings, remove shoots by clipping so that only one individual remains in each container.
- Allow 2-3 months for plant growth and CMN establishment.
5. Establishment of experimental plants and treatments
- Sow experimental plants by seeding or transplanting into containers. If seeding, wait until all containers have a germinated seedling before removing pre-treatment nurse plants by clipping their shoots. If transplanting, clip all pre-treatment plants before transplanting experimental seedlings to prevent unintended competitive effects.
- Establish CMN treatments by either leaving the containers not moved for the duration of the experiment (intact CMNs) or rotating them weekly to physically sever hyphae extending among the modified containers (severed CMNs; Figure 1A). When severing CMNs, rotate each container through one full rotation to avoid unintentionally altering aboveground interactions, particularly for heliotropic plants.
- Heavily water all pots or microcosms immediately after rotation to reestablish contact between the interstitial substrate and the sides of containers.
6. Tracing of mineral nutrient movement across CMNs
- Fertilize neighboring plants with 0.5% 15N enriched KNO3 and NH4Cl.
- Fertilize the target individual with a 14N fertilizer of equal concentration.
7. Monitoring and maintenance of experiment
- Regularly (at least monthly) re-randomize the positions of the pots or microcosms over the course of the experiment.
- Weekly measure growth, such as height or longest leaf length (for grasses) to monitor when growth begins to slow, because it is important to harvest before the plants become root-bound.
8. Harvest of the experiment
- Clip all aboveground tissue and place individual plants into labeled envelopes that identify their treatment, pot or microcosm, and position.
- Dry aboveground tissues at 60 °C to constant weight. Measure the dry weight of each plant tissue.
- Allow the soil to dry before extracting the containers and harvesting the roots.
- Delicately brush off as much soil as possible from the root systems and wash them in a pan of water or under a gentle stream of water on a sieve of 250 micron pore size.
- Allow the roots to air-dry and weigh the whole root system.
- Clip the root system haphazardly and store the root fragments in 50% ethanol. After they are stained33, use these fragments for quantification of root colonization using the gridline intersection method34.
- Re-weigh the remaining root system and store it in a labeled paper envelope to dry at 60 °C for assessment of dry weight. Use the following equation to calculate the weight of the entire root system:
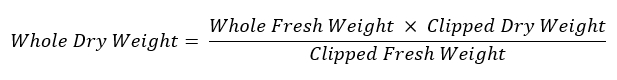
9. Mineral nutrient and stable isotope analyses
- Group the seedlings by biomass into “deciles” or 10 groups, “octiles” or 8 groups, “quartiles” or four groups, etc. after rank-ordering them by weight if the tissue quantity is too low for minimum requirements for digestion to determine mineral nutrient concentrations.
- Send foliar samples to a contracted lab for mineral nutrient and stable isotope analyses (Table of Materials).
- Describe isotopic abundance using the following customary expression:
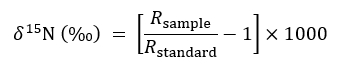
where R represents the 15N/14N ratio of a sample or of the standard which is atmospheric N. - Use the non-modified, solid container treatment to serve as a control for background 15N ratios in the following mass balance equation when quantifying the amount of 15N taken up by a target plant in severed or intact CMN treatments:
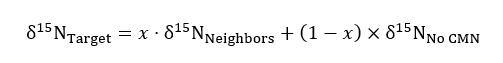
where δ15N represents the isotopic abundance of targets, neighbors, and target plants in the no CMN treatment, and x represents (as a decimal fraction) the percent nitrogen obtained by the target plant from neighbor containers to which label was added. Values for δ15NNeighbors are obtained for each target plant’s composited neighbors.
- Describe isotopic abundance using the following customary expression:
Access restricted. Please log in or start a trial to view this content.
Results
To determine how CMNs may influence plant performance through nutrient partitioning, we grew Andropogon gerardii Vitman, a dominant prairie grass, in a target plant experiment with 6 equally spaced neighbors and intact, severed, or no CMNs. We found that severing or preventing CMNs diminished targets’ aboveground dry weights (Figure 2), suggesting that intact CMNs promoted plant growth. Plants with severed CMNs and prevented CMNs responded nota...
Access restricted. Please log in or start a trial to view this content.
Discussion
Our results affirm that our rotated core method can sharply focus on the role of CMNs in belowground plant interactions. There are several critical steps in the protocol, however, that if altered, have potential to influence the ability to detect CMN effects. It is critical to fill the interstitial area surrounding containers with a nutrient-poor medium. In our unsuccessful, rotated-core target plant experiment with guava tree seedlings, although there was a marked reduction of target growth in the presence of any number...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank the two anonymous reviewers for their suggestions. We also thank the numerous undergraduates who have helped with constructing pots, microcosms, and slotted containers and who have assisted with maintaining and harvesting experiments. We also thank North Central College for startup funds (to JW) and current facilities, as well as Ashley Wojciechowski for obtaining a North Central College Richter Grant supporting an experiment using these methods. Part of this work was funded by a National Science Foundation Doctoral Dissertation Improvement Grant (DEB-1401677).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Commercial tubular seedlings container (called 'containers' in the manuscript) | Stuewe and Sons, Inc | Ray Leach Cone-tainer ™ | RLC3U |
| Course glass beads | Industrial Supply, Inc. | 12/20 sieve | Size #1 |
| Course silica sand | Florida Silica Sand | 6/20 50lb bags | None |
| Fine glass beads | Black Beauty | Black Beauty FINE Crushed Glass Abrasive (50 lbs) | BB-Glass-Fine |
| Hydrophobic membrane | Gore-tex | None | None |
| Large commercial tubular seedling containers | Stuewe and Sons, Inc. | Deepot ™ | D16L |
| Medium silica sand | Florida Silica Sand | 30/65 50 lb bags | None |
| Nylon mesh | Tube Lite Company, Inc. | Silk screen | LE7-380-34d PW YEL 60/62 SEFAR LE PECAP POLYESTER |
| Soil and foliar nutrient analysis facility | Kansas State University Soil Testing Lab | None | None |
| Stable isotope core facility | University of Miami | None | None |
References
- Remy, W., Taylor, T. N., Hass, H., Kerp, H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proceedings of the National Academy of Sciences of the United States of America. 91 (25), 11841-11843 (1994).
- Smith, S. E., Read, D. J. Mycorrhizal Symbiosis. , 3 edn, Academic Press. (2008).
- Giovannetti, M., Avio, L., Sbrana, C. Mycorrhizal Networks. , Springer. 41-67 (2015).
- Giovannetti, M., Sbrana, C. Cell Biology of Plant and Fungal Tip Growth. Vol. 328. Nato Science Series, Sub-Series I: Life and Behavioural Sciences. Cresti, A., Heath, M., Geitmann, I. B. , 221-231 (2001).
- Giovannetti, M., Sbrana, C., Avio, L., Strani, P. Patterns of below-ground plant interconnections established by means of arbuscular mycorrhizal networks. New Phytologist. 164 (1), 175-181 (2004).
- Avio, L., Pellegrino, E., Bonari, E., Giovannetti, M. Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mycelial networks. New Phytologist. 172 (2), 347-357 (2006).
- Giovannetti, M., et al. At the root of the wood wide web: self recognition and nonself incompatibility in mycorrhizal networks. Plant Signaling & Behavior. 1 (1), 1-5 (2006).
- Miller, R., Kling, M. J. The importance of integration and scale in the arbuscular mycorrhizal symbiosis. Plant and Soil. 226 (2), 295-309 (2000).
- Miller, R., Jastrow, J., Reinhardt, D. R. External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia. 103 (1), 17-23 (1995).
- Weremijewicz, J., Sternberg, L. dS. L. O., Janos, D. P. Common mycorrhizal networks amplify competition by preferential mineral nutrient allocation to large host plants. New Phytologist. , (2016).
- Fellbaum, C. R., et al. Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytologist. 203 (2), 646-656 (2014).
- Lekberg, Y., Hammer, E. C., Olsson, P. A. Plants as resource islands and storage units--adopting the mycocentric view of arbuscular mycorrhizal networks. FEMS Microbiol Ecol. 74 (2), 336-345 (2010).
- Jakobsen, I., Hammer, E. C. Mycorrhizal Networks. , Springer. 91-131 (2015).
- Jakobsen, I., Rosendahl, L. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytologist. 115 (1), 77-83 (1990).
- Kiers, E. T., et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science. 333 (6044), 880-882 (2011).
- Hartnett, D. C., Hetrick, B. A. D., Wilson, G. W. T. Mycorrhizal influence on intra- and interspecific neighbour interactions among co-occuring prairie grasses. Journal of Ecology. 81 (4), 787-795 (1993).
- Hetrick, B. A. D., Wilson, G. W. T., Todd, T. C. Differential responses of C3 and C4 grasses to mycorrhizal symbiosis, phosphorus fertilization, and soil microorganisms. Canadian Journal of Botany. 68 (3), 461-467 (1990).
- Johnson, D., Leake, J. R., Read, D. J. Novel in-growth core system enables functional studies of grassland mycorrhizal mycelial networks. New Phytologist. 152 (3), 555-562 (2001).
- Leake, J. R., et al. Networks of power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Canadian Journal of Botany-Revue Canadienne De Botanique. 82 (8), 1016-1045 (2004).
- Babikova, Z., et al. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecology Letters. 16 (7), 835-843 (2013).
- Schüepp, H., Miller, D. D., Bodmer, M. A new technique for monitoring hyphal growth of vesicular-arbuscular mycorrhizal fungi through soil. Transactions of the British Mycological Society. 89 (4), 429-435 (1987).
- Miller, D. D., Bodmer, M., Schüepp, H. Spread of endomycorrhizal colonization and effects on growth of apple seedlings. New Phytologist. 111 (1), 51-59 (1989).
- Jakobsen, I., Gazey, C., Abbott, L. K. Phosphate transport by communities of arbuscular mycorrhizal fungi in intact soil cores. New Phytologist. 149 (1), 95-103 (2001).
- Johnson, D., Leake, J., Ostle, N., Ineson, P., Read, D. J. In situ 13CO2 pulse‐labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytologist. 153 (2), 327-334 (2002).
- Johnson, D., Leake, J., Read, D. J. Transfer of recent photosynthate into mycorrhizal mycelium of an upland grassland: short-term respiratory losses and accumulation. of 14C. Soil Biology and Biochemistry. 34 (10), 1521-1524 (2002).
- Walder, F., et al. Mycorrhizal networks: Common goods of plants shared under unequal terms of trade. Plant Physiology. 159 (June 2012), 789-797 (2012).
- Song, Y. Y., et al. Interplant communication of tomato tlants through underground common mycorrhizal networks. Plos One. 5 (10), e13324(2010).
- Merrild, M. P., Ambus, P., Rosendahl, S., Jakobsen, I. Common arbuscular mycorrhizal networks amplify competition for phosphorus between seedlings and established plants. New Phytologist. 200 (1), 229-240 (2013).
- Janos, D. P., Scott, J., Aristizábal, C., Bowman, D. M. J. S. Arbuscular-mycorrhizal networks inhibit Eucalyptus tetrodonta seedlings in rain forest soil microcosms. Plos One. 8 (2), e57716(2013).
- Wyss Lozano Hoyos, T. Pinus elliottii var. densa Seedling Performance Reflects Ectomycorrhizas, Soil Nutrient Availability and Root Competition. , (2010).
- Mäder, P., Vierheilig, H., Alt, M., Wiemken, A. Boundries between soil compartments formed by microporous hydrophobic membranes (GORE-TEX) can be crossed by vesicular-arbuscular mycorrhizal fungi but not by ions in the soil solution. Plant and Soil. 152, 201-206 (1993).
- Mäder, P., et al. Transport of 15N from a soil compartment separated by a polytetrafluoroethylene membrane to plant roots via the hyphae of arbuscular mycorrhizal fungi. New Phytologist. 146 (1), 155-161 (2000).
- Brundrett, M., Bougher, N., Dell, B., Grove, T. Working with Mycorrhizas in Forestry and Agriculture. , (1996).
- McGonigle, T. P., Miller, M. H., Evans, D. G., Fairchild, G. L., Swan, J. A. A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytologist. 115 (3), 495-501 (1990).
- Weremijewicz, J., Janos, D. P. Common mycorrhizal networks amplify size inequality in Andropogon gerardii monocultures. New Phytologist. 198 (1), 203-213 (2013).
- Weremijewicz, J., O’Reilly, L. dS. L., Janos, D. P. Arbuscular common mycorrhizal networks mediate intra-and interspecific interactions of two prairie grasses. Mycorrhiza. , 1-13 (2017).
- Weiner, J. Asymmetric competition in plant populations. Tree. 5 (11), 360-364 (1990).
- Damgaard, C., Weiner, J. Describing inequality in plant size or fecundity. Ecology. 81 (4), 1139-1142 (2000).
- Johnson, C. R., Joiner, J. N., Crews, C. E. Effects of N, K, and Mg on growth and leaf nutrient composition of 3 container grown woody ornamentals inoculated with mycorrhizae. Journal of the American Society for Horticultural Science. 105 (2), 286-288 (1980).
- Estrada-Luna, A. A., Davies, F. T., Egilla, J. N. Mycorrhizal fungi enhancement of growth and gas exchange of micropropagated guava plantlets (Psidium guajava L.) during ex vitro acclimatization and plant establishment. Mycorrhiza. 10 (1), 1-8 (2000).
- Hurlbert, S. H. Pseudoreplication and the design of ecological field experiments. Ecological Monographs. 54 (2), 187-211 (1984).
- Argüello, A., et al. Options of partners improve carbon for phosphorus trade in the arbuscular mycorrhizal mutualism. Ecology Letters. 19 (6), 648-656 (2016).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved