A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Accelerating Rate Calorimetry and Complementary Techniques to Characterize Battery Safety Hazards
* These authors contributed equally
In This Article
Summary
A method to characterize the potential failure hazards of lithium batteries is achieved with accelerating rate calorimetry. Heat and pressure release, visual observation of the failure event, and the capture of evolved gases are collected in this experiment to identify the worst credible threats of batteries taken to failure.
Abstract
The hazards associated with lithium-based battery chemistries are well-documented due to their catastrophic nature. Risk is typically qualitatively assessed through an engineering risk matrix. Within the matrix, potentially hazardous events are categorized and ranked in terms of severity and probability to provide situational awareness to decision makers and stakeholders. The stochastic nature of battery failures, particularly the lithium-ion chemistry, makes the probability axis of a matrix difficult to properly assess. Fortunately, characterization tools exist, such as accelerated rate calorimetry (ARC), that characterize degrees of battery failure severity. ARC has been used extensively to characterize reactive chemicals but can provide a new application to induce battery failures under safe, controlled experimental conditions and quantify critical safety parameters. Due to the robust nature of the extended volume calorimeter, cells may be safely taken to failure due to a variety of abuses: thermal (simple heating of cell), electrochemical (overcharge), electrical (external short circuit), or physical (crush or nail penetration). This article describes the procedures to prepare and instrument a commercial lithium-ion battery cell for failure in an ARC to collect valuable safety data: onset of thermal runaway, endotherm associated with polymer separator melting, pressure release during thermal runaway, gaseous collection for analytical characterization, maximum temperature of complete reaction, and visual observation of decomposition processes using a high temperature borescope (venting and cell can breach). A thermal “heat-wait-seek” method is used to induce cell failure, in which the battery is heated incrementally to a set point, then the instrument identifies heat generation from the battery. As heat generates a temperature rise in the battery, the calorimeter temperature follows this temperature rise, maintaining an adiabatic condition. Therefore, the cell does not exchange heat with the external environment, so all heat generation from the battery under failure is captured.
Introduction
Rechargeable batteries, specifically lithium-ion chemistry, have allowed functioning of an all-electric society encompassing all aspects of daily life such as transportation, communication, and entertainment. For these energy storage applications, charge capacity equates to range or runtime. Maximizing these parameters leads to aggressively high energy lithium-ion cells. Unfortunately, as electrical energy increases within lithium-ion cells, so does detrimental energy release when a failure occurs1. A number of regulatory agencies, professional societies, and independent laboratories have developed standards to better characterize the safety of rechargeable batteries. One method used to quantify the thermal intensity of a battery safety event is accelerated rate calorimetry (ARC)2,3. This type of calorimetry is performed near-adiabatically to capture explicit heat generation from a material or battery cell at the onset of an exothermic reaction, then through thermal runaway and combustion type reaction processes. The ARC instrument provides an opportunity to characterize the worst-case heat, pressure, and gas generation from an exothermic material reaction in a safe and controlled laboratory environment.
The ARC instrument was first developed in the 1970s to simulate exothermic runaway reactions from hazardous and reactive chemicals at safe scales and evaluate the hazards of reactive chemicals to devise safety procedures for handling, usage, storage, and transportation4. In the early 1980s, ARC was first used for the purpose of studying thermal runaway reactions in lithium cells. The ARC operates through “adaptive adiabatic control”, which means the calorimeter temperature tries to match the cell temperature while a reaction is occurring. There is also no heat exchange between the sample being tested and the surrounding environment. In doing so, as the cell self-heats and its temperature rises, heat transfer between the cell and its surroundings is minimized. A schematic of the ARC chamber with heating elements and locations for lithium-ion cell testing is shown in Figure 1.
The ARC instrument is available in several sizes to accommodate a wide range of battery materials, cell components, cells, batteries, and battery modules, as shown in Table 1. The ARC also offers a range of thermal analysis testing protocols, including the most prevalent for lithium-ion battery safety characterization known as heat-wait-seek (HWS). ARC measurements can be performed in an “open” or “closed” testing configuration. The main difference between these two testing configurations is the ability to perform pressure and gas sampling measurements in the closed system. The open configuration lends itself to visual observation through use of a high temperature camera or borescope4,5. The use of a small spherical pressure vessel or “bomb” has been utilized in the ARC to measure reaction heat release from battery electrode materials6. Typically, heat release is governed by the lithium concentration in the materials and intensifies in the presence of organic electrolyte solvents and lithium salts7,8. At the cellular level, an extended volume ARC is required to safely retain the heat, pressure, and gas release from the thermal runaway process. Additionally, features can be incorporated into the ARC instrument to induce battery failures via nail penetration, electrochemical overcharge, or external short circuit.
Sandia National Laboratory has historically been a leader in ARC characterization of batteries in support of the U.S. Departments of Energy and Transportation. Sandia has published many reports highlighting its importance in generating critical safety data, which has influenced federal policy and safety standards9,10. In the report, they provide optimal test parameters, data collection, and reporting criteria9. Most of the recommended practices are adopted in this article to characterize the thermal hazard of a single cylindrical lithium-ion cell under thermal runaway utilizing the HWS protocol. Specifically, the ARC can provide objective quantitative evidence of factors affecting the safety of lithium-ion batteries and battery materials (i.e., maximum temperature, heating rate as function of time/temperature, vent gas as a function of time/temperature, and chemical analysis of hazardous substances from vented gas and smoke) during a battery failure.
The most commonly used ARC testing protocol for battery safety testing is HWS. The HWS protocol offers accurate detection of exothermic reactions occurring within lithium-ion cells and is more accurate than a simple ramped heating mode. This is the standard method for battery thermal runaway characterization. The chamber is heated to an initial start temperature, then a wait time is applied that depends upon the sample mass and heat transfer properties. After this step, the calorimeter seeks for an exotherm greater than the set sensitivity (e.g., 0.02 °C/min). If no exotherm is observed in the allotted time period, the chamber again heats by a defined temperature step (e.g., 5 °C), and the process is repeated. Figure 2 shows the process flowchart for HWS (Figure 2A) and experimental data illustrating the various stages of HWS through the first several iterations (Figure 2B).
Complete definitions of each of the testing steps in the HWS protocol are as follows. Heat mode is the power given to chamber heaters to elevate chamber and device under test (DUT) temperature. Wait mode occurs when thermal equilibrium is established between the calorimeter and bomb or test article. Seek mode occurs when calculations of change in temperature are determined, and the time relates to the change in sensitivity, typically 0.02 °C/min. Cool mode is initiated at the end of a test, when a maximum temperature or pressure has been achieved. The traditional cooling mechanism involves flowing an inert gas such as nitrogen into the chamber. Alternatively, liquid nitrogen may be introduced into the chamber to expedite cooling. Exotherm mode refers to an increase in temperature observed after a seek step is termed exotherm. This describes an environment in which self-heating of the test article is greater than the selected sensitivity, typically 0.02 °C/min. Exotherm mode continues until the rate of self-heating falls below the desired sensitivity, at which point another heat mode is triggered, and the heat-wait-seek sequence continues until a maximum temperature or pressure limit is reached.
Protocol
1. Calibration of calorimeter
NOTE: It is important to calibrate the calorimeter to accommodate any changes in heat transfer conditions to/from the same cell (e.g., connecting large diameter electrical cables to the cell) or replacement of the main measurement thermocouple. The instrument should be recalibrated after a period of 2–3 months, as thermocouple responses can change with prolonged use.
- Use a small spherical vessel or “bomb” for calibration of the calorimeter.
- Attach an empty spherical bomb of known material (i.e., titanium, stainless steel, aluminum, etc.) to the underside of the calorimeter lid.
- Ensure that the calorimeter is clean and free of debris.
- Match the calibration conditions to the anticipated testing conditions. Any special fixtures must be present within the chamber in the anticipated location for proper calibration.
- Connect the tip of the bomb thermocouple wire to the surface of the spherical bomb vessel. The tip must be in contact with the bomb in order for the calibration to work correctly. Secure the thermocouple wire and leads with high temperature tape, if necessary.
- Ensure that the calorimeter lid is completely closed, with the lid and base showing good contact.
- Close the blast box to eliminate air currents blowing across the calorimeter, which can affect the measurement.
- Use the following parameters for a calibration test: temperature step = 25 °C; start temperature = 50 °C; end temperature = 405 °C; temperature rate sensitivity = 0.01 °C/min; and wait time = 30 min.
- Ensure that the previous calibration offsets are cleared from the software.
- Begin the calibration procedure.
2. Phi factor test
NOTE: Even the highest performing ARC cannot achieve full adiabicity. Therefore, some heat is lost during the test and must be accounted for to provide accurate calorimetry data.
- Account for thermal loss by calculating an offset factor, ϕ, using the following equation:

Apply the known heat capacity and mass (c and m) for the bomb and sample. Complete a drift test after calibration of the chamber. Ensure that the resulting phi factor is within ±0.02 °C/min.
3. Heat mass and heat capacity of commercial battery cells for destructive testing
- Calculate the heat capacity during a short, mild, nondestructive heating of the cell. Perform this operation in a temperature range of 25–55 °C (ambient temperature to the maximum recommended operating temperature of the cell). Use liquid nitrogen to evaluate heat capacity from sub-ambient temperatures.
- Collect the single cell mass for three identical cells.
- Affix a heater mat along the axis of a single 18650 cell with high temperature tape.
NOTE: The physical test set-up can vary with cell geometry, and a heater mat of suitable size is required for different cell sizes. - For extended volume calorimeters, bundle three cells, including the cell with heater mat, into a triangle shape. Tape the cells together with aluminum tape.
- Attach a control thermocouple at the mid-length of a cell adjacent to the cell with the heater mat.
- Suspend the three-cell triangle from the top of the calorimeter using metal wire.
- Securely replace the calorimeter lid.
- Ensure wires from heater exit the calorimeter and are connected to the variable power supply.
- Initiate the heat capacity test by activating the power supply to ramp from 30–60 °C over a period of ~2 h.
NOTE: Typical temperature vs. time data (converted to K/s) used to calculate cp is provided in Figure 3. The power supplied to the heater is calculated by multiplying the power supply voltage and current to provide the power in units of W or J/s. The heater power is divided by the slope of the temp vs. time plot to provide the thermal mass in units of J/K. Finally, the thermal mass is divided by the sample mass to provide the cell heat capacity in units of J/g·K. An example of the heat capacity measurement is shown below, according to data in Figure 3:
Slope of temperature vs. time, from raw data: 0.3738 °C/min = 0.00623 K/s
Power from heater: (8.53 V x 0.639 A) @ 30% = 1.635 W = 1.635 J/s
Thermal mass (power/slope) = 262.472 J/K
Heat capacity (thermal mass/mass) = 262.472 J/K divided by 244 g = 1.075 J/g·K
4. Destructive failure testing a commercial 18650 lithium-ion battery cell
- Standard “heat-wait-seek” for battery cell
- Ensure that the commercial battery/cell test article or “device under test” (DUT) is at the desired state of charge (SOC) for testing; ideally, the SOC is 100% to represent the “worst credible threat” of a battery failure.
- Open the lid of the outer chamber.
- Remove the top lid of the calorimeter to arrange for placement of the battery test article. The chamber should be clear of debris using a standard vacuum and light solvent wipe of the calorimeter walls.
- Mount the cylindrical cell into a cell holder in the vertical direction and place it slightly off-center of the calorimeter’s interior. Off-center placement ensures maximum video capture during the thermal runaway event when the high temperature borescope camera is not obstructed by ejected electrolyte vapors, smoke, and cell ejecta from the cell’s top vent.
NOTE: Alternatively, the cell may be fixed in a horizontal direction using a standard ring-stand. Anytime that additional items such as ring stands are entered into the calorimeter, another calibration should be performed. - Affix the thermocouple designated “bomb thermocouple” to the cylindrical cell at the wall mid-length and secured with high temperature nickel wire. This is done to 1) keep the thermocouple in place during mechanical straining of the cell can and 2) avoid melting of alternative high temperature tape, which sometimes cannot withstand the degree of heat release from the cell.
NOTE: It is important to maintain good contact between the thermocouple and cell wall to ensure the accurate temperature reading required to control adiabatic heating of the calorimeter chamber. - Secure the DUT with appropriate alligator style clips for cell charge, discharge, open circuit voltage monitoring, or electrochemical impedance measurements. Run the electrical leads through the grooves on the top surface of the calorimeter chamber.
- Replace the calorimeter lid careful not to pinch any thermocouples or electrical leads.
- Use the manual focus features on the high temperature borescope to maximize picture quality prior to testing. Often the borescope is focused on the bottom plate of the cell holder to account for fluctuations in optical focus during heating of the calorimeter.
- Initiate a thermal HWS testing protocol. The testing parameters and representative values are as follows:
- Start temperature: 35 °C
- End temperature: 305 °C
- Temperature step: 5 °C
- Temperature rate sensitivity: 0.02 °C/min
- Wait time: 30 min
- Calculation temperature step: 0.2 °C
- Cool temperature: 35 °C
- Release temperature: 50 °C
- Safety pressure: 200 Bar
- Maximum temperature drop: 25 °C
- Maximum pressure drop: 20 Bar
- Max exotherm rate: 1000.00 °C/min
- Maximum pressure rate: 160342 Bar/min
- Data log temperature step: 1.00 °C
- Data log time step: 0.5 min
- Exotherm log temperature step: 1.00 °C - If gas collection is desired, set the collection temperature (e.g., 120 °C) and collection time period (e.g., 0.5 min).
- Initiate the HWS test and allow the cell to enter thermal runaway.
NOTE: Once a maximum temperature of the calorimeter is reached, an exhaust fan is automatically initiated to remove any smoke from the calorimeter. - Allow the chamber to completely cool to near ambient temperature before opening the ARC and removing the calorimeter lid. Cooling time for the chamber may be expedited using liquid or gaseous nitrogen injection into the bottom of the chamber. Without nitrogen-assist, cooling may take up to 24 h.
- The ARC HWS process results in the decomposition/combustion of the battery cell, leaving combusted electrode materials and debris inside the chamber. Clean the calorimeter using a shop vacuum and wipe the walls of the calorimeter with a mild solvent.
5. Ensuring successful ARC test of lithium-ion cell
- Ensure that the cell is at the appropriate SOC. Fully charged cells typically provide the greatest heat release and earliest onset temperature, indicating the worst credible safety threat.
- Ensure that the bomb thermocouple is secured to cell with metal wire. Failure to adhere the thermocouple tip to the battery sidewall will not capture the effects of self-heating.
- Double-check the thermocouple designations: bomb is attached to cell, sample is free floating within the calorimeter chamber, and (if using multiple auxiliary thermocouples) their locations are known and verified.
- If performing open circuit voltage monitoring or performing electrochemistry in the ARC, make sure that the cell registers an expected voltage value. Unexpected voltage or negative voltage suggests that the electrical leads within the ARC canister may have lost connection or the leads have been reversed. Be careful not to short the cell during set-up, as the whole chamber is metallic.
6. Interpreting ARC data and calculating heat of reaction
- Calculate the total heat of reaction in units of heat per mass (J/g or J/kg).
- Use temperature vs. time data to obtain basic thermal properties of the reaction, such as the onset of exotherm, Tonset, and maximum temperature of the reaction, Tmax, using the equation:
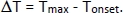
- Use the heat capacity measured in the previous procedure and calculation of ΔT to calculate the total heat of reaction. Use the ϕ offset factor to account for lack of perfect adiabicity.
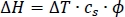
- Calculate the pressure rise during reaction using the following equation:
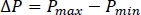
- Plot the logarithmic temperature rate vs. temperature to show how the reaction develops across the temperature range (Figure 4B). Convert the temperature rate (°C/min) into units of J/s using the heat capacity.
Results
Representative data from the HWS experiment of a fully charged 18650 commercial lithium-ion battery cell is provided in Figure 4A,B. The figure shows cell temperature as a function of time during a “closed” ARC testing set-up. Basic thermal features (Tonset, Tmax, and ΔT) are highlighted in the figure. The location of Tonset is the beginning of the exothermic step, which continues until Tmax is reached. Cell voltag...
Discussion
The HWS testing procedure accomplished with the ARC instrument is critical to determining the worst credible safety threat posed by a lithium-ion battery. The measurements of self-heat onset temperature and maximum temperature during thermal runaway provide the necessary objective data to accurately assess the safety of lithium-ion cells. Through the use of ARC-based experiments, battery safety metrics can be measured in a controlled and reproducible manner.
One limitation of the ARC instrumen...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Mr. Danny Montgomery from Thermal Hazard Technology for his many insightful comments and suggestions. The authors thank the Office of Naval Research and Department of Transportation-Pipeline and Hazardous Materials Safety Administration for funding support and procurement of the accelerating rate calorimeter.
Materials
| Name | Company | Catalog Number | Comments |
| borescope | Optronics | Rigid, high temperature borescope | |
| Energy Lab Potentiostat | Princeton Applied Research / Ametek | potentiostat capable of collecting open circuit voltage, galvanostic/potentiostatic battery cycling and electrochemical impedance spectroscopy | |
| Extended Volume Accelerating Rate Calorimeter | Thermal Hazard Technologies | Mid-sized system, sample range: components to batteries. Working volume: 0.57 m3 | |
| high temperature tape | non specific | ||
| lithium-ion battery cell | various | rechargeable mixed metal oxide versus graphite lithium-ion cell in 18650 form factor | |
| mat heater | Omega | form factor and size dependent upon battery cell for heat capacity measurements | |
| spherical bomb | Thermal Hazard Technologies | small volume bomb for calibration of ARC |
References
- Love, C. T. Perspective on the Mechanical Interaction Between Lithium Dendrites and Polymer Separators at Low Temperature. Journal of Electrochemical Energy Conversion and Storage. 13 (3), (2016).
- Doughty, D. H., Roth, E. P. A General Discussion of Li Ion Battery Safety. The Electrochemical Society Interface. 21 (2), 37-44 (2012).
- Waldmann, T., et al. Electrochemical, Post-Mortem, and ARC Analysis of Li-Ion Cell Safety in Second-Life Applications. Journal of The Electrochemical Society. 164 (13), 3154-3162 (2017).
- Lei, B., et al. Experimental Analysis of Thermal Runaway in 18650 Cylindrical Li-ion Cells using an Accerlerating Rate Calorimeter. Batteries. 3 (14), (2017).
- von Sacken, U., Nodwell, E., Sundher, A., Dahn, J. R. Comparative thermal stability of carbon intercalation anodes and lithium metal anodes for rechargeable lithium batteries. Journal of Power Sources. 54, 240-245 (1995).
- Richard, M. N., Dahn, J. R. Accelerating rate calorimetry study on the thermal stability of lithium intercalated graphite in electrolyte I. Experimental Journal of The Electrochemical Society. 146 (6), 2068-2077 (1999).
- Richard, M. N., Dahn, J. R. Predicting electrical and thermal abuse behaviours of practical lithium-ion cells from accelerating rate calorimeter studies on small samples in electrolyte. Journal of Power Sources. 79 (2), 135-142 (1999).
- Orendorff, C. J., Lamb, J., Steele, L. A. M. . Recommended Practices for Abuse Testing Rechargeable Energy Storage Systems (RESSs). , (2017).
- Orendorff, C. J., et al. . Advanced Inactive Materials for Improved Lithium-Ion Battery Safety. , 74 (2012).
- Lampe-Onnerud, C., Shi, J. H., Singh, S. K., Barnett, B. Fourteenth Annual Battery Conference on Applications and Advances. Proceedings of the Conference (IEEE). , 215-220 (1999).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved