A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Leukocyte Infiltration of Cremaster Muscle in Mice Assessed by Intravital Microscopy
In This Article
Summary
Here, we show how to perform intravital microscopy on post-capillary venules of the mouse cremaster muscle. Commonly applied to different models of inflammation and sepsis, particularly those induced by chemokines and cytokines, we highlight its relevance in the study of muscolopathies involving exaggerated muscular leukocyte infiltration.
Abstract
Intravital microscopy (IVM) is widely used to monitor physiological and pathophysiological processes within the leukocyte recruitment cascade in vivo. The current protocol represents a practical and reproducible method to visualize the leukocyte endothelium interaction leading to leukocyte recruitment in skeletal muscle derived tissue within the intact organism of the mouse. The model is applicable to all fields of research that focus on granulocyte activation and their role in disease.
We provide a step by step protocol to guide through the method and to highlight potential pitfalls and technical difficulties. The protocol covers the following aspects: experimental settings and required material, anesthesia of the mouse, dissection of the cremaster muscle as well as tracheal and carotid cannulation, IVM recordings and offline analysis. Data formats like adherent leukocytes, rolling flux (RF) and rolling flux fraction (RFF) are explained in detail and appropriate applications are discussed. Representative results from dystrophin deficient mdx mice are provided in the results section.
IVM is a powerful tool to assess leukocyte recruitment in an in vivo setting; however, delineating for example endothelial and leukocyte function may require a combination with ex vivo setups like flow chamber experiments. Furthermore, the genetic background of animals of interest may greatly influence baseline recruitment, requiring individual fine tuning of the protocol provided. Despite its limitations, IVM may serve as a platform to readily translate in vitro findings into a living vertebrate organism.
Introduction
Intravital microscopy (IVM) is a commonly applied tool in the field of leukocyte biology. Leukocyte recruitment follows a cascade of well-defined events initiated by leukocyte capture, rolling and adhesion to the endothelial wall, and finally transmigration and extravasation of leukocytes to the actual site of inflammation1. Each step is mediated and controlled by various chemokines (e.g., IL-8/CXCL8), receptors (e.g., LFA-1, Mac-1) and corresponding endothelial cell adhesion molecules (e.g., ICAM-1, VCAM-1 and E-Selectin)2,3. The interaction of different regulatory sites, controlling factors and mediators of the leukocyte recruitment cascade like receptor of advanced glycation end products (RAGE), intercellular adhesion molecule 1 (ICAM-1), C-X-C motif ligand (CXCL)1/2 and their receptor CXCR2 were uncovered using IVM4,5,6,7,8,9.
The method of IVM has been described for many different organs and tissues such as the intestine10, skin11, lymph nodes12, the embryonic yolk sack13 and others. However, the most widely studied method of IVM is the cremaster model, first described in rats14. Whilst still used in rats15, the method is nowadays mainly applied in mice due to the high abundance of different transgenic lines. Our group has recently highlighted the potential role of cremaster IVM in the field of inflammatory muscolopathies like Duchenne Muscular Dystrophy (DMD) studying dystrophin-deficient mdx mice16. Due to its thin interwoven and easily accessible fiber composition, the cremaster muscle represents the ideal candidate muscle to be studied as a whole mount using light or fluorescent microscopy. Leukocyte recruitment and extravasation mainly take place in post-capillary venules, which can readily be identified on a continuous muscular layer in the cremaster muscle.
The advantage of in vivo imaging compared to other in vitro assays is its biological context in a living organism. At the same time, delineating cell-specific contributions to altered leukocyte recruitment may require additional in vitro models like flow chambers or endothelial assays. The combination of multiple methods will yield most convincing data. Scientists should be aware of the limitations of the cremaster model as any surgical manipulation will lead to increased leukocyte trafficking and recruitment. Hence, baseline recruitment is difficult to estimate with this method. Despite its broad application, IVM of the cremaster can be challenging and a novel setup may take time and resources to establish. We now provide an easy protocol which will help to avoid some of the common mistakes in IVM. Also, limitations will be discussed and complimentary methods will be highlighted where applicable.
IVM of the cremaster represents an ideal approach to be implemented in the field of inflammatory and infectious studies. More specifically, the cremaster model may be of high interest to scientists studying skeletal muscle biology in the context of inflammatory disease.
Protocol
Animals were housed under controlled and specific pathogen-free conditions at the IBF (Interfakultäre Biomedizinische Forschungseinrichtung), Heidelberg. All the procedures described here were approved by the local IRB and the Regierungspraesidium Karlsruhe, Baden-Wuerttemberg, Germany.
1. Anesthesia administration
- Anesthetize the mouse by intraperitoneal (i.p.) bolus injection of 125 mg/kg ketamine and 12.5 mg/kg xylazine.
- Place and fix the mouse in a dorsal recumbent position on a heating pad to maintain body temperature of the mouse (36.5-38 °C). Use a non-absorbable sterile suture (6/0) to wind a simple loop around the frontal teeth and tape the joining ends of the suture to the heating pad. This fixation aims to keep the mouth open in order to avoid disturbance in breathing.
- Check the mouse for appropriate depth of anesthesia by interdigital toe pinch (withdrawal should not be seen).
- Once sufficient anesthesia is reached proceed to surgical preparation. Repeatedly confirm anesthesia along the ongoing experiment every 30 min. Re-administer drugs as described above if needed.
- Equilibrate physiological salt solution (PSS, composition: 132 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgS04, 2.0 mmol/L CaCl2, 18 mmol/L NaHCO3) with 5% CO2/95% N2 for 15 minutes. Continuously bubble PSS with 5% CO2/95% N2 throughout the experiment and keep at 37 °C.
2. Surgical preparation of the trachea and carotid artery (optional)
- Dissect the skin from the neck area by gently pulling the skin with tweezers in the midline. Use scissors to apply a circular cut of 1-2 cm in diameter to give enough space for surgical preparation.
- Carefully dissect the surrounding muscle, fat and connective tissues using tweezers.
- Make a transverse cut (~ 1.3 mm) into the trachea using small surgical scissors and introduce a polyethylene tube (I.D x O.D. 0.034" x 0.050") inside the caudal end of the trachea to secure the upper airways.
- Fix the tube located in the trachea by a single circular knot suture (6/0 USP).
- Locate the carotid artery along the right side of trachea and dissect the surrounding tissue from the carotid artery wall. Alternatively, the jugular or also the tail vein could be used for i.v. access. Try to avoid injury to the vagal nerve, as it is located closely.
- Pass two pieces of suture (6/0) underneath the carotid artery. Place the first cranial suture proximal, close to the bifurcation of carotid arteries and tie it permanently. The second suture will be located about 5-8 mm distal from the first one and later be used to secure the tube in the carotid artery. Do not tie it yet.
- Prepare a polyethylene tube (I.D x O.D. 0.011" x 0.024") with a length of 30 cm by bending the end part to a 1 mL syringe needle filled with saline (0.9% NaCl). Rigorous flushing is important to prevent air embolization during preparation.
- Use a 7 mm vessel clip to clamp the carotid artery distally of the second suture.
- Perform a small transverse cut (~0.5 mm) in the carotid artery and introduce the sterile polyethylene tube. Secure the tube in the artery using the second suture prepared before.
- Remove the vessel clip and gently apply a little tension to the syringe connected to the polyethylene tube. This carotid catheter can now be used to administer drugs or take blood samples if required, even monitoring of blood pressure or pulse rate is possible with respective devices.
3. Surgical preparation of the cremaster muscle
- Transfer the mouse on the heating pad (along with the heating pad) to the custom-made plastic frame holding the stage for later microscopy. Orient the mouse with its scrotum facing the microscope stage.
- Reassess depth of anesthesia before proceeding; re-administer a quarter to half dose of anesthesia if required.
- Localize the scrotum, holding it at its most distal part using tweezers, pull it gently and cut off a circular section of scrotal skin with about 5 mm in diameter. Make sure not to harm the underlying structures such as the cremaster muscle. Make sure to keep the open tissue well hydrated with saline solution.
- Dissect loose connective tissue using two tweezers and localize both testes.
- Hold one testis distally and gently start pulling it out, removing surrounding connective tissue step by step.
- Once the testis is exteriorized, pin its distal end to the rubber ring surrounding the stage. Upon exteriorization, hydrate the tissue with saline solution during the whole process.
- Critical step: Carefully remove connective tissue without harming the underlying cremaster muscle. Excess connective tissue may obstruct vision in later microscopy and may produce blurry images.
- Pin the distal part of the testis down and open the cremaster muscle by a small transverse cut (about 1 mm), followed by longitudinal incision from the very distal to the proximal end. As a result the cremaster muscle should open spherically. Macroscopically visible vessel should not be severed, as this may impact hemodynamics.
- Carefully spread the muscle over the glass stage and pin it to the rubber ring. Make sure not to touch or harm the central region, as microscopy will be performed here.
Caution: Excess stretch may obstruct blood flow. - Pin the remaining testis aside to give access to the region of interest. Make sure to moisten with PSS repeatedly to prevent drying. The mounted muscle is now ready for microscopy.
4. Intravital microscopy
- Place the mounted cremaster muscle in the upright microscope and perform microscopy using a 40x objective.
- Acquire data and export using high throughput imaging spectrography.
- Perform recordings under continuous superfusion with preheated (35-37 °C) PSS17. Apply superfusion by a small tubing that has been taped to the upright objective of the microscope to allow continuous dripping of PSS alongside the objective down onto the muscle tissue.
- Identify post-capillary venules (confluence of two smaller vessels) and measure the microcirculation on venules with a diameter of 20-40 μm.
- Record high-resolution images of the microcirculation from the cremaster muscle in a time range of 30 s. As there might be slight contraction and relaxation of the muscle tissue during recording, make sure to continuously adjust the focus. Generally, the mouse tends to exhibit stable circulation for about 2 hours. It is possible to acquire several recordings of different vessels from different regions. One or both testis can be used subsequently.
NOTE: As this is a model of inflammation, common ways of induction are: systemic application or local scrotal injection of pro inflammatory mediators as well as superfusion with for example N-Formylmethionyl-leucyl-phenylalanine (fMLP). Local TNF-α stimulation is commonly used to trigger leukocyte recruitment; LPS-induced endotoxemia is a model of SIRS severe systemic inflammation.
5. Leukocyte visualization
NOTE: Adherent and rolling leukocytes can easily be seen without further visualization. To determine the center line velocity of freely moving leukocytes, perform differential staining.
- If eGFP labeled mice are used, directly analyze them by fluorescence microscopy.
- For non-eGFP labeled mouse strains, use rhodamine staining.
- Administer rhodamine 6G at 0.2 mg/kg body weight. Repeated doses may be required to achieve sufficient staining intensities. Make sure to keep small volumes, as larger volumes may affect hemodynamic parameters.
- For instant staining of leukocytes, administer the stain via the carotid artery. If carotid artery dissection has not been performed, sufficient staining may be achieved by i.p. application using the same dose18. As an alternative to carotid artery catheterization, any other venous catheterization can be performed.
NOTE: Keep in mind that rhodamine staining, unlike eGFP labeling, shows time decay of fluorescence intensity as well as an overall staining maximum of about 80% of leukocytes at higher concentrations. - Visualize freely moving rhodamine stained leukocytes by fluorescence microscopy.
6. End of experiment
NOTE: The overall estimated time to perform this protocol should be 90 – 150 minutes.
- End the experiment with euthanasia by cervical dislocation.
- For further analyses, such as transmigration, fix the cremaster muscle in PFA whilst still stretched on the stage and stained for histology as required.
7. Offline analysis
- Analyze the following parameters as simple counts during video playback.
- Calculate rolling flux [number/min]: number of rolling cells passing an imaginary line/min
- Calculate adhesion [number/mm2]: number of cells adhesive to the vessel wall within the field of view ≥ 30 s/vascular surface [mm2].
- Measure the following hemodynamic and microvascular parameters are measured using ImageJ.
- Calculate vessel diameter [µm] as the inner wall diameter.
- Calculate vessel length [µm] as the center line length of the vessel.
- Calculate center line velocity
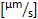 obtained from frame-to-frame video analysis of freely moving leukocytes in center line.
obtained from frame-to-frame video analysis of freely moving leukocytes in center line.
- Use the following equations as an estimation.
- Calculate vascular surface in [mm2]: vessel length [µm] x vessel diameter [µm] x π x 10-6.
- Calculate wall shear rate
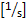 : 4.9 x (8 x center line velocity
: 4.9 x (8 x center line velocity 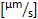 x 0.625/vessel diameter [µm]).
x 0.625/vessel diameter [µm]). - Calculate mean blood flow velocity
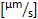 : center line velocity
: center line velocity 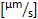 x 0.625.
x 0.625. - Calculate total leukocyte flux
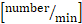 : systemic leukocyte count
: systemic leukocyte count 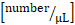 x (mean blood flow velocity
x (mean blood flow velocity 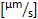 x 60/1000) x π x
x 60/1000) x π x 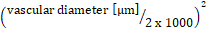 .
. - Calculate rolling flux fraction [%]: rolling flux/total leukocyte flux x 100.
Results
IVM as per the provided protocol will yield unique insights into the cascade of leukocyte recruitment in skeletal muscle. The results section will focus on typical results obtained by IVM and highlight potential problems that may encounter.
The experimental setup for intravital microscopy is outlined in Figure 1. Preparation of the cremaster muscle and removal of connective tissue is crucial to obtain focused microscopic images with a uniform surface. Excess conne...
Discussion
IVM as a method has been widely used to study different cell types in different organs and has been extensively described and discussed19. The main aim of this study is to provide an efficient approach to set up and perform IVM in the cremaster muscle. Practicing the method will produce reliable and reproducible results. Thus, planning and standardization are key factors to master the technique. Above all, the technique is very dependent on hemodynamic and microvascular parameters, which need to b...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the German Federal Ministry of Education and Research (BMBF) 01GL1746E as part of the PRIMAL Consortium. The authors acknowledge Britta Heckmann and Silvia Pezer for skillful technical assistance.
Materials
| Name | Company | Catalog Number | Comments |
| Material | |||
| Ketanest S | Pfizer Pharma GmbH | PZN: 08509909 | anesthesia. Generic / IUPAC Name: ketamine |
| Xylazine | CP-Pharma GmbH | Article-nr.: 1205 | anesthesia. Generic / IUPAC Name: xylazine (as hidrochloride) |
| Saline Solution | B. Braun Melsungen | PZN 02737756 | surgical preparation. Generic / IUPAC Name: sodium chloride |
| Syringe needle Omnican F | B. Braun Melsungen | REF 9161502 | surgical preparation |
| Suture 6/0 USP | Resorba | REF 4217 | surgical preparation |
| Polyethylene tube #10 | BD GmbH | Supplier No. 427401 | surgical preparation |
| Polyethylene tube #90 | BD GmbH | Supplier No. 427421 | surgical preparation |
| Rhodamine 6G | Sigma-Aldrich Chemie GmbH | CAS Number 989-38-8 | leukocyte staining. Generic / IUPAC Name: ethyl 2-[3-(ethylamino)-6-ethylimino-2,7-dimethylxanthen-9-yl]benzoate |
| Setup Equipment | |||
| Upright microscope | Olympus | BX51W1 | microscopy |
| 40-fold objective | Zeiss | Achroplan 40 × /0.80 W | microscopy |
| ImSpector software | Lavision Biotec GmbH | ver. 4.0.469 | software |
| ImageJ | National Institute of Health, USA | ver. 1.51j8 | software |
References
- Ley, K., Laudanna, C., Cybulsky, M. I., Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews. Immunology. 7 (9), 678-689 (2007).
- Zanardo, R. C. O., et al. A down-regulatable E-selectin ligand is functionally important for PSGL-1-independent leukocyte-endothelial cell interactions. Blood. 104 (12), 3766-3773 (2004).
- Woodfin, A., et al. ICAM-1-expressing neutrophils exhibit enhanced effector functions in murine models of endotoxemia. Blood. 127 (7), 898-907 (2016).
- Frommhold, D., et al. RAGE and ICAM-1 cooperate in mediating leukocyte recruitment during acute inflammation in vivo. Blood. 116 (5), 841-849 (2010).
- Braach, N., et al. RAGE controls activation and anti-inflammatory signalling of protein C. PloS One. 9 (2), 89422 (2014).
- Frommhold, D., et al. RAGE and ICAM-1 differentially control leukocyte recruitment during acute inflammation in a stimulus-dependent manner. BMC Immunology. 12 (1), 56 (2011).
- Braach, N., et al. Anti-inflammatory functions of protein C require RAGE and ICAM-1 in a stimulus-dependent manner. Mediators of Inflammation. 2014, 743678 (2014).
- Girbl, T., et al. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity. 49 (6), 1062-1076 (2018).
- Smith, M. L., Olson, T. S., Ley, K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. The Journal of Experimental Medicine. 200 (7), 935-939 (2004).
- Emre, Y., Jemelin, S., Imhof, B. A. Imaging Neutrophils and Monocytes in Mesenteric Veins by Intravital Microscopy on Anaesthetized Mice in Real Time. Journal of Visualized Experiments. (105), (2015).
- Eriksson, E., Boykin, J. V., Pittman, R. N. Method for in vivo microscopy of the cutaneous microcirculation of the hairless mouse ear. Microvascular Research. 19 (3), 374-379 (1980).
- von Andrian, U. H. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation. 3 (3), 287-300 (1996).
- Hudalla, H., et al. LPS-induced maternal inflammation promotes fetal leukocyte recruitment and prenatal organ infiltration in mice. Pediatric Research. 84 (5), 757-764 (2018).
- Grant, R. T. Direct observation ok skeletal muscle blood vessels (rat cremaster). The Journal of Physiology. 172 (1), 123-137 (1964).
- Thiele, J. R., Goerendt, K., Stark, G. B., Eisenhardt, S. U. Real-time digital imaging of leukocyte-endothelial interaction in ischemia-reperfusion injury (IRI) of the rat cremaster muscle. Journal of Visualized Experiments. (66), e3973 (2012).
- Kranig, S. A., et al. Dystrophin deficiency promotes leukocyte recruitment in mdx mice. Pediatric Research. 11, 4457 (2019).
- Bagher, P., Segal, S. S. The mouse cremaster muscle preparation for intravital imaging of the microcirculation. Journal of Visualized Experiments. (52), e2874 (2011).
- Reichenbach, Z. W., Li, H., Gaughan, J. P., Elliott, M., Tuma, R. IV and IP administration of rhodamine in visualization of WBC-BBB interactions in cerebral vessels. Microscopy Research and Technique. 78 (10), 894-899 (2015).
- Secklehner, J., Lo Celso, C., Carlin, L. M. Intravital microscopy in historic and contemporary immunology. Immunology and Cell Biology. 95 (6), 506-513 (2017).
- Nussbaum, C., et al. Neutrophil and endothelial adhesive function during human fetal ontogeny. Journal of Leukocyte Biology. 93 (2), 175-184 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved