A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Retinal Vascular Reactivity as Assessed by Optical Coherence Tomography Angiography
In This Article
Summary
This article describes a method for measuring retinal vasculature reactivity in vivo with human subjects using a gas breathing provocation technique to deliver vasoactive stimuli while acquiring retinal images.
Abstract
The vascular supply to the retina has been shown to dynamically adapt through vasoconstriction and vasodilation to accommodate the metabolic demands of the retina. This process, referred to as retinal vascular reactivity (RVR), is mediated by neurovascular coupling, which is impaired very early in retinal vascular diseases such as diabetic retinopathy. Therefore, a clinically feasible method of assessing vascular function may be of significant interest in both research and clinical settings. Recently, in vivo imaging of the retinal vasculature at the capillary level has been made possible by the FDA approval of optical coherence tomography angiography (OCTA), a noninvasive, minimal risk and dyeless angiography method with capillary level resolution. Concurrently, physiological and pathological changes in RVR have been shown by several investigators. The method shown in this manuscript is designed to investigate RVR using OCTA with no need for alterations to the clinical imaging procedures or device. It demonstrates real time imaging of the retina and retinal vasculature during exposure to hypercapnic or hyperoxic conditions. The exam is easily performed with two personnel in under 30 min with minimal subject discomfort or risk. This method is adaptable to other ophthalmic imaging devices and the applications may vary based on the composition of the gas mixture and patient population. A strength of this method is that it allows for an investigation of retinal vascular function at the capillary level in human subjects in vivo. Limitations of this method are largely those of OCTA and other retinal imaging methods including imaging artifacts and a restricted dynamic range. The results obtained from the method are OCT and OCTA images of the retina. These images are amenable to any analysis that is possible on commercially available OCT or OCTA devices. The general method, however, can be adapted to any form of ophthalmic imaging.
Introduction
The metabolic demand of the retina is dependent on an adequate and constant supply of oxygen provided by a well-regulated system of arterioles, capillaries and venules1. Several studies have demonstrated that the function of larger caliber human retinal vessels can be assessed in vivo with various physiologic2,3,4,5 and pharmacologic6,7 stimuli. In addition, abnormal function of this vascular system is common in retinal vascular diseases such as diabetic retinopathy where retinal vascular reactivity (RVR) has been shown to be attenuated even in its earliest stages8,9 through both gas provocation9 and flickering light experiments5,10,11. Retinal vascular risk factors such as smoking have also been correlated with impaired RVR12 and retinal blood flow13. These findings are important since the clinical symptoms of retinal vascular disease occur relatively late in the disease process and proven early clinical markers of disease are lacking14. Thus, assessing RVR can provide useful measures of vascular integrity for the early assessment of abnormalities that can initiate or exacerbate retinal degenerative diseases.
Previous RVR experiments have usually relied upon devices such as a laser blood flowmeter9 or fundus cameras equipped with special filters15 for retinal image acquisition. However, these technologies are optimized for larger diameter vessels such as arterioles16 and venules15, which are not where gas, micronutrient and molecular exchange occur. A more recent study was able to quantify the RVR of capillaries using adaptive optics imaging17, but despite the improved spatial resolution, these images have a smaller field size and are not FDA approved for clinical use18.
The recent advent of optical coherence tomography angiography (OCTA) has provided an FDA approved, noninvasive and dyeless angiographic method of assessing capillary level changes in human patients and subjects in vivo. OCTA is widely accepted in clinical practice as an effective tool for assessing impairment in capillary perfusion in retinal vascular diseases such as diabetic retinopathy19, retinal venous occlusions20, vasculitis21 and many others22. OCTA therefore provides an excellent opportunity for the evaluation of capillary level changes, which can have significant spatial and temporal heterogeneity23 as well as pathologic changes, in a clinical setting. Our group recently demonstrated that OCTA can be used to quantify the responsiveness of retinal vessels at the capillary level2 to physiologic changes in inspired oxygen, which is a retinal vasoconstrictive stimulus16,24, and carbon dioxide, which is a retinal vasodilatory stimulus3,5.
The goal of this article is to describe a protocol that will allow the reader to assess the retinal vascular reactivity of the smaller arterioles and capillary bed using OCTA. The methods are adapted from those presented in Lu et al.25 who described the measurement of cerebrovascular reactivity with magnetic resonance imaging. Although the present methods were developed and used during OCTA imaging2, they are applicable to other retinal imaging devices with relatively simple and obvious modifications.
Access restricted. Please log in or start a trial to view this content.
Protocol
This study was approved by the University of Southern California Institutional Review Board and adhered to the tenets of the Declaration of Helsinki.
1. Setup of Gas Non-rebreathing Apparatus
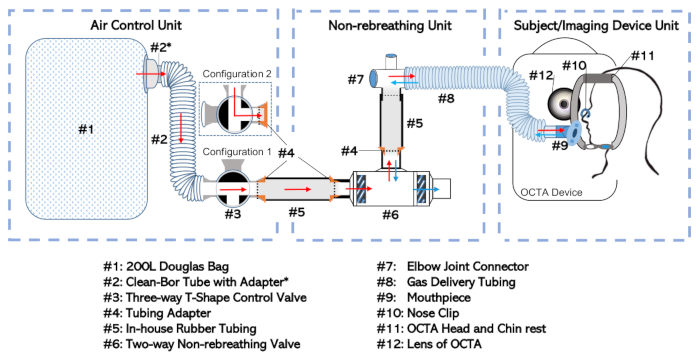
Figure 1: Diagram of the non-rebreathing apparatus. The full setup has been broken into three separate units according to their function and the frequency with which they are dealt with independently. These include: the Air-Control Unit, the Non-rebreathing Unit, and the Subject/Imaging Device Unit Please click here to view a larger version of this figure.
- Apparatus assembly
- Connect the Douglas bag (Figure 1, #1) to the three-way valve (#3) at a selective inlet port via the 35 mm inner-diameter tube (#2; see Table of Materials) with adapter (#2*). This combination will be called the “Air Control Unit” as shown in Figure 1.
- Connect the two-way non-rebreathing valve (#6) to the elbow joint connector (#7) at the non-rebreathing valve’s mouth port. Form the connection using a rubber tube (#5) fitted with an adapter (#4).
- Connect the elbow joint to the gas delivery tubing (#8). This setup, including the non-rebreathing valve (#6), in-house tubing (#5), adapters (#4), elbow joint (#7), and gas delivery tubing (#8) will be called the “Non-rebreathing Unit”.
NOTE: Minimize the amount of dead space between the subject’s mouth and the diaphragm of the two-way non-rebreathing valve (#6). - Connect the Air Control Unit at the outlet port of the three-way valve (#3) to the Non-rebreathing Unit at the inlet port of the two-way non-rebreathing valve (#6). Make the connection using additional rubber tubing (#5) and adapters (#4) as those described earlier that allow the pieces to be inserted into one another.
- Seal all loose connections by wrapping the joints with sealing tape to ensure a hermetic fit.
- Connect the gas delivery tubing (#8) at its open end to a mouthpiece (#9) as shown in the Subject/Imaging Device Unit of Figure 1.
NOTE: This step (1.1.6) can be deferred until the subject testing is ready to begin (Step 3.5).
- Preparation of the Air Control Unit for gas non-rebreathing
- Isolate the Air Control Unit by disconnecting it from any in-house tubing (#5) or adapters (#4) if it is not already separated.
- Ensure the Douglas bag (#1) is empty or empty the Douglas bag (#1) of any air by systematically rolling-up the bag from the distal end towards the bag’s inlet port with the three-way valve (#3) set to Configuration 1 as shown in Figure 1.
- Fill the Douglas bag (#1) with the appropriate gas mixture.
- If only room-air non-rebreathing is intended, set the three-way valve to Configuration 2 (shown in Figure 1) and do not fill the Douglas bag (#1). Otherwise continue with the steps that comprise Step 1.2.3.
- Connect the Air Control Unit (shown in Figure 1) at the outlet port of the three-way valve (#3) to a gas-cylinder (containing the desired air-mixture) using the appropriate adapters and tubing. Use a cuff adapter to mount a 1/8” gas filling tube to the outer diameter of the three-way valve (#3).
- Set the three-way valve assembly to Configuration 1 (as shown in Figure 1) to allow the intended gas to flow from the storage cylinder into the Douglas bag (#1). Open the gas cylinder.
- Once the Douglas bag (#1) is filled to the intended volume (usually half-filled), close the gas cylinder outlet and set the three-way valve to Configuration 2, which isolates the gas within the Douglas bag (#1). Disconnect the Air Control Unit from any tubing used to fill the Douglas bag (#1).
2. Preparing the Subject for Imaging
- After the subject consents to participate in the study, sit the subject behind the OCTA imaging device. Explain the testing procedures to the subject.
- Confirm the subject’s medical history to ensure that the subject has no existing medical conditions that increase the risk of participating in the study.
NOTE: Pre-existing cardiovascular or pulmonary diseases are risk factors for which subjects may be excluded from participating. It is essential that the subject understand that they can stop the procedure at any time for any reason such as feeling lightheaded or some additional unexpected discomfort. - Determine the eye to be assessed as per the testing protocol. One eye only may be imaged to limit the testing time and minimize the potential discomforts from the gas non-rebreathing.
- Consider eye dilation if the subject has a pupil size of about 2.5 mm or less. Although dilation is not mandatory, it enhances the chances of acquiring good quality images. To dilate, instill one drop each of 0.5% proparacaine hydrochloride ophthalmic solution, 1% tropicamide ophthalmic solution and 2.5% phenylephrine hydrochloride ophthalmic solution. Full dilation should occur within 10–15 min.
3. Gas Provocation Experiment and Image Acquisition
- Create a profile for the patient in the OCTA machine.
- Wear gloves.
- Wipe down the OCTA head and chin rest with an alcohol swab to disinfect the setup.
- Free the mouthpiece (#9) from its sterile packaging.
NOTE: Refrain from touching the mouthpiece as much as possible as this component makes direct contact with the mucus lining of the mouth of the subject - Connect the mouthpiece (#9) to gas delivery tubing (#8)
- Place a pulse oximeter on the subjects’ finger and begin monitoring oxygen saturation levels and pulse.
NOTE: Once the subject begins breathing the desired air mixture, the pulse oximeter should be continuously monitored by the examiner. If the oxygen saturation of the subject drops below 94%, the experiment should be stopped, as a safety precaution, and the subject observed until they return to baseline. - Adjust the height of the OCTA setup so that the subject can easily rest their chin on the chinrest (#11) without overextending or flexing their neck.
- Loop the gas delivery tubing (#8) with mouthpiece (#9) attachment through the head and chin rest with the mouthpiece (#9) facing the patient. Have the tubing loop through the machine oppposite the side of the eye that the subject is having imaged.
- Insert the mouthpiece into the patient’s mouth. Encourage the subject to practice breathing through the non-rebreathing setup to create familiarity with the apparatus. Ensure the subject takes deep breathes to facilitate gas exchange.
- Place the nose clip (#10) on the subject to ensure they are breathing through the mouthpiece.
- Keep the three-way valve on Configuration 2 or change it to Configuration 1 depending on whether images are being acquired for exposure to room air or a specific gas mixture, respectively. For future reference, note the time as the start of gas inhalation.
- Have the subject place their chin on the right or left section of the chinrest (#11) according to the eye selected for imaging.
- Ensure they move their head forward until their forehead is in firm contact with the headrest (#11).
- Capture the OCTA scan of interest as determined by the testing protocol. In this study, three 3 mm x 3 mm images centered on the fovea were captured after 1 min of gas breathing.
- Have the subject keep their head facing forward and still while fixating on the target in the center of their view
- In the live image seen in the iris view, center the scan.
- Bring the iris into focus by moving the chinrest in or out using the left-right arrows.
- Make sure the foveal dip is centered in the OCT scan, which should occur by default.
- Take an image. Scanning will usually last several seconds on an OCTA machine.
- View the OCTA image after the completion of the scan and ensure it is of adequate quality. Signal strength should be a 7 or better on a 10-point scale provided by the OCTA manufacturer.
- Select save or rescan the eye.
- Repeat steps 3.14.1–3.14.7 for as many scans are desired.
- Allow the subject to sit back from the machine. Remove the nose clip (#10) and the mouthpiece (#9) when no more scans of the eye with this gas mixture are needed.
- Allow subjects a 2 min break before starting CO2 gas provocation experiments.
- Fill the Douglas bag with the first desired air mixture (consisting of 5% CO2, 21% oxygen and 74% nitrogen) as specified in step 1.2. The three-way valve will be in Configuration 2 after this step.
- Complete gas non-rebreathing apparatus setup by connecting the Air Control Unit to the Non-rebreathing Unit as shown in Figure 1 and described in step 1.1.4. Make sure all joints are airtight with sealing tape.
- Repeat steps 3.9–3.14, but now set the three-way valve to Configuration 1 when directed in step 3.11.
- Give subjects a 10 min break after the CO2 gas provocation to allow a return to baseline.
- While the subject is on break, fill the Douglas bag with 100% O2 according to step 1.2.
- Repeat steps 3.17–3.18 to perform the experiment under 100% O2 gas provocation conditions.
4. Experimental Clean Up
- Discard the disposable elements of the setup: the subject’s mouthpiece (#9) and nose clip (#10).
- Clean the head and chin rest (#11) using an alcohol swab. Wipe the subject chair, OCTA table and OCTA handles with a disinfectant wipe to remove any errant saliva.
- Disconnect the setup into its base components—the Air Control Unit and Non-rebreathing Unit—at the three-way valve (#3).
- As no air exhaled from the subject should have reached the elements of the Air Control Unit, empty the Douglas bag according to step 1.2.2 and place in a location for future retrieval. Disconnect the clean-bor tube (#2) with adapter (#2*) and three-way valve (#3) from the Douglas bag if desired for easier storage. This completes the Air Control Unit clean up.
- Remove the gas delivery tubing (#8) from the Non-rebreathing Unit by disconnecting it from the elbow joint (#7). Disconnect the in-house rubber tubing (#5) and tubing adapters (#4), from the two-way non-rebreathing valve (#6). Then do the same from the elbow joint (#7) by removing the sealing tape and detaching the parts by pulling them apart.
NOTE: More extensive cleaning of the two-way non-rebreathing valve may be facilitated by disassembling it to remove the internal diaphragms for additional care. - Prepare a disinfectant bath for cleanup of the reusable components
- Fill a container large enough to submerge the gas delivery tubing (#8) with an appropriately diluted and well mixed detergent disinfectant. In this case, dilute the detergent with water to a ratio of 1:6425.
- Soak the gas delivery tubing (#8), two-way non-rebreathing valve (#6), elbow joint (#7), in-house rubber tubing (#5) and tubing adapters (#4) in the prepared disinfectant bath for at least 10 min.
- Remove all parts after the bath is over and rinse them thoroughly with water.
- Place them on a paper towel on a clean countertop to be air-dried.
- Once air drying has completed, dispose of the paper towel and place all components away for storage.
5. OCTA Data Export and Analysis
- OCTA data export
- Export OCTA data by inserting a removable media device of choice into the OCTA computer. Find the subject and scan of interest.
- Select Export to create a zip folder containing the subject of interest’s data in a .bmp format on the removable media device.
- OCTA data analysis
- Organize the OCTA data on a laboratory computer with the ability to perform additional image analysis and processing.
- Use a custom script to suppress noise with a global thresholding technique and perform additional feature extraction. Binarize and skeletonize the OCTA images.
- On the post-processed images, calculate the vessel skeleton density (VSD)19,26, a dimensionless measure of the total linear length of vessels in an image calculated by the following equation performed on a binarized skeletonized image of the OCTA:
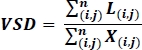
where i and j refer to pixel coordinate (i,j), L(i,j) refers to white pixels representing decorrelation, X(i,j) refers to all pixels, and n refers to the dimensions of the pixel array, which can be assumed to be n x n pixels19,26. The denominator of this equation represents the total number of pixels which is calculated as written from the skeletonized image, but can be thought of as representing the physical area of the entire image.
Access restricted. Please log in or start a trial to view this content.
Results
The output from this experiment consists of the manual readings taken from the pulse oximeter, the timing noted for gas exposure or OCTA scanning and the raw OCTA imaging data. An OCTA image consists of the OCT B-scans and the decorrelation signal associated with each B-scan. The data parameters are given by the specifications of the device. A swept source laser platform OCTA machine with a central wavelength of 1040–1060 nm was used. The images provide a transverse resolution of 20 µm and optical axial resolu...
Access restricted. Please log in or start a trial to view this content.
Discussion
The methodology just described is the complete protocol for a gas breathing provocation experiment that allows for the measurement of a subject’s RVR in a controlled environment at specific timepoints with no modifications to the OCTA imaging device and minimal discomfort or risk to the subject. This setup is described in a way that allows for easy modifications to fit the needs of the researcher. It can accommodate additional tubing to fit different clinic rooms and certain elements such as the in-house tubing or ...
Access restricted. Please log in or start a trial to view this content.
Disclosures
Carl Zeiss Meditec has provided grant funding, equipment and financial support to AHK related to the topic of this article.
Acknowledgements
This work was supported by NIH K08EY027006, R01EY030564, UH3NS100614, Research Grants from Carl Zeiss Meditec Inc (Dublin, CA) and Unrestricted Department Funding from Research to Prevent Blindness (New York, NY).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 5% CO2 gas [5% CO2, 21% O2, 74% N2] (Compressed) | Institution Dependent (Praxair) | ||
| Bacdown Disinfectant Detergent | Decon Labs | 8001 | https://deconlabs.com/products/disinfectant-bdd/ |
| Clean-Bor Tubes (35 mm Inner Diameter) | Vacumed | 1011-108 | http://www.vacumed.com/zcom/product/Product.do?compid=27&skuid=1197 |
| Cuff adapter for Douglas bag filling | Vacumed | 22254 | http://www.vacumed.com/zcom/product/Product.do?compid=27&prodid=343 |
| Douglas bag (200 L capacity) | Harvard Apparatus | 500942 | https://www.harvardapparatus.com/douglas-bag.html |
| Elbow Joint (Inner Diameter 19 mm/Outer Diameter 22 mm), Modified in House | |||
| Fingertip Pulse Oximeter (Pro-Series) | CMS | CMS 500DL | https://www.walmart.com/ip/Pro-Series-CMS-500DL-Fingertip-Pulse-Oximeter-Blood-Oxygen-Saturation-Monitor-with-silicon-cover-batteries-and-lanyard/479049154 |
| Gas Delivery Tube (22 mm Inner Diameter) Modified in House | |||
| Gas filling tube (1/8" for compressed gas) | |||
| Hydrogen Peroxide Cleaner Disinfectant Wipes | Clorox Healthcare | 30824 | https://www.cloroxpro.com/products/clorox-healthcare/hydrogen-peroxide-cleaner-disinfectants/?gclid=EAIaIQobChMIk-KG4vi15QIVcRh9Ch0NNwLPEAAYASAAEgJIa_D_BwE&gclsrc=aw.ds |
| Lubricant Eye Drops | Refresh | Refresh Plus | https://www.refreshbrand.com/Products/refresh-plus |
| Manual Directional Control Valves: Three-Way T-Shape Stopcock Type (Inner Diameter 28.6 mm, Outer Diameter 35 mm) | Hans Rudolph | 2100C Series | www.rudolphkc.com |
| Medical O2 (Compressed) | Institution Dependent | ||
| Mouth piece (Silicone, Model #9061) | Hans Rudolph | 602076 | www.rudolphkc.com |
| OCTA Imaging Device (PLEX Elite 9000) | Carl Zeiss Meditec, Dublin, CA, USA | https://www.zeiss.com/meditec/int/product-portfolio/optical-coherence-tomography-devices/plex-elite-9000-swept-source-oct.html | |
| Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% | Paragon Bioteck, Inc | NDC 42702-102-15 | https://paragonbioteck.com/products/diagnostics/phenylephrine-hydrochloride-ophthalmic-solution-usp-2-5/ |
| Plastic Nose Clip Sterile Foam CS100 | Sklar Sterile | 96-2951 | https://www.sklarcorp.com/disposables/plastic/plastic-nose-clip-sterile-foam-box-of-100.html |
| Proparacaine Hydrochloride Ophthalmic Solution, USP .5% | Bausch + Lomb | NDC 24208-730-06 | https://www.bausch.com/ecp/our-products/rx-pharmaceuticals/generics |
| Regulator (tank dependent- 5% CO2: Fisherbrand Mulitstage Gas Cylinder Regulators) | Genstar Technologies Company | 10575150 | https://www.fishersci.com/shop/products/fisherbrand-multistage-cylinder-regulators-22/10575150?keyword=true |
| Regulator (tank dependent- Oxygen: Fisherbrand Multistage Gas Cylinder Regulators) | Genstar Technologies Company | 10575145 | https://www.fishersci.com/shop/products/fisherbrand-multistage-cylinder-regulators-22/10575145?keyword=true |
| Rubber Tubing (Inner diameter 19 mm, Outer diameter 27 mm), Made in House | |||
| Sealing tape- Parafilm Wrap (2" Wide) | Cole Parmer | PM992 | https://www.coleparmer.com/i/parafilm-pm992-wrap-2-wide-250-ft-roll/0672050?PubID=VV&persist=True&ip=no&gclid=EAIaIQobChMInY3vqomz5QIVfyCtBh1VSg64EAAYASAAEgJ9n_D_BwE |
| Sterile Alcohol Prep Pads | Medline | MDS090670 | https://www.medline.com/product/Sterile-Alcohol-Prep-Pads/Swab-Pads/Z05-PF03816 |
| Tropicamide Ophthalmic Solution, USP 1% | Akorn | NDC 17478-102-12 | http://www.akorn.com/prod_detail.php?ndc=17478-102-12 |
| Tubing Adapter, Made in House | |||
| Two-way non-rebreathing valve (2600 Series- Inner Diameter 28.6 mm, Outer Diameter 35 mm) | Hans Rudolph | 2600 Series, UM-112078 | www.rudolphkc.com |
References
- Country, M. W. Retinal metabolism: A comparative look at energetics in the retina. Brain Research. 1672, 50-57 (2017).
- Ashimatey, B. S., Green, K. M., Chu, Z., Wang, R. K., Kashani, A. H. Impaired Retinal Vascular Reactivity in Diabetic Retinopathy as Assessed by Optical Coherence Tomography Angiography. Investigative Ophthalmology & Visual Science. 60 (7), 2468(2019).
- Hickam, J. B. M. D., Frayser, R. P. D. Studies of the Retinal Circulation in Man: Observations on Vessel Diameter, Arteriovenous Oxygen Difference, and Mean Circulation Time. Circulation. 33 (2), 302-316 (1966).
- Dorner, G. T., Garhoefer, G., Zawinka, C., Kiss, B., Schmetterer, L. Response of Retinal Blood Flow to CO2 -Breathing in Humans. European Journal of Ophthalmology. 12 (6), 459-466 (2002).
- Linsenmeier, R. A., Zhang, H. F. Retinal oxygen: from animals to humans. Progress in Retinal and Eye Research. 58, 115-151 (2017).
- Eliakim, M., Mor, I., Michaelson, I. C. Assessment of pharmacologic effects on the retinal circulation of hypertensive subjects by a quantitative method. Microvascular Research. 4 (4), 374-383 (1972).
- Gilmore, E. D., et al. Retinal arteriolar hemodynamic response to an acute hyperglycemic provocation in early and sight-threatening diabetic retinopathy. Microvascular Research. 73 (3), 191-197 (2007).
- Hickam, J. B., Sieker, H. O. Retinal Vascular Reactivity in Patients with Diabetes Mellitus and with Atherosclerosis. Circulation. 22 (2), 243-246 (1960).
- Gilmore, E. D., et al. Retinal Arteriolar Diameter, Blood Velocity, and Blood Flow Response to an Isocapnic Hyperoxic Provocation in Early Sight-Threatening Diabetic Retinopathy. Investigative Ophthalmology & Visual Science. 48 (4), 1744(2007).
- Garhofer, G. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. British Journal of Ophthalmology. 88 (7), 887-891 (2004).
- Felder, A. E., Wanek, J., Blair, N. P., Shahidi, M. Inner Retinal Oxygen Extraction Fraction in Response to Light Flicker Stimulation in Humans. Investigative Ophthalmology & Visual Science. 56 (11), 6633-6637 (2015).
- Rose, K., Flanagan, J. G., Patel, S. R., Cheng, R., Hudson, C. Retinal Blood Flow and Vascular Reactivity in Chronic Smokers. Investigative Ophthalmology & Visual Science. 55 (7), 4266(2014).
- Omae, T., Nagaoka, T., Yoshida, A. Effects of Habitual Cigarette Smoking on Retinal Circulation in Patients With Type 2 Diabetes. Investigative Ophthalmology & Visual Science. 57 (3), 1345(2016).
- Pusparajah, P., Lee, L. H., Abdul Kadir, K. Molecular Markers of Diabetic Retinopathy: Potential Screening Tool of the Future. Frontiers in Physiology. 7, (2016).
- Hammer, M., Vilser, W., Riemer, T., Schweitzer, D. Retinal vessel oximetry-calibration, compensation for vessel diameter and fundus pigmentation, and reproducibility. Journal of Biomedical Optics. 13 (5), 054015(2008).
- Gilmore, E. D., Hudson, C., Preiss, D., Fisher, J. Retinal arteriolar diameter, blood velocity, and blood flow response to an isocapnic hyperoxic provocation. American Journal of Physiology-Heart and Circulatory Physiology. 288 (6), 2912-2917 (2005).
- Duan, A., Bedggood, P. A., Metha, A. B., Bui, B. V. Reactivity in the human retinal microvasculature measured during acute gas breathing provocations. Scientific Reports. 7 (1), 2113(2017).
- Burns, S. A., Elsner, A. E., Sapoznik, K. A., Warner, R. L., Gast, T. J. Adaptive optics imaging of the human retina. Progress in Retinal and Eye Research. 68, 1-30 (2019).
- Kim, A. Y., Chu, Z., Shahidzadeh, A., Wang, R. K., Puliafito, C. A., Kashani, A. H. Quantifying Microvascular Density and Morphology in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography Angiography. Investigative Ophthalmology & Visual Science. 57 (9), (2016).
- Koulisis, N., et al. Quantitative microvascular analysis of retinal venous occlusions by spectral domain optical coherence tomography angiography. PLOS ONE. 12 (4), 0176404(2017).
- Kim, A. Y., et al. Quantifying Retinal Microvascular Changes in Uveitis Using Spectral-Domain Optical Coherence Tomography Angiography. American Journal of Ophthalmology. 171, 101-112 (2016).
- Kashani, A. H., et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Progress in Retinal and Eye Research. 60, 66-100 (2017).
- Yu, D. Y., et al. Retinal capillary perfusion: Spatial and temporal heterogeneity. Progress in Retinal and Eye Research. 70, 23-54 (2019).
- Tayyari, F., et al. The Relationship between Retinal Vascular Reactivity and Arteriolar Diameter in Response to Metabolic Provocation. Investigative Ophthalmology & Visual Science. 50 (10), 4814(2009).
- Lu, H., Liu, P., Yezhuvath, U., Cheng, Y., Marshall, O., Ge, Y. MRI Mapping of Cerebrovascular Reactivity via Gas Inhalation Challenges. Journal of Visualized Experiments. (94), e52306(2014).
- Reif, R., Qin, J., An, L., Zhi, Z., Dziennis, S., Wang, R. Quantifying Optical Microangiography Images Obtained from a Spectral Domain Optical Coherence Tomography System. International Journal of Biomedical Imaging. 2012, 1-11 (2012).
- Olafsdottir, O. B., Eliasdottir, T. S., Kristjansdottir, J. V., Hardarson, S. H., Stefánsson, E. Retinal Vessel Oxygen Saturation during 100% Oxygen Breathing in Healthy Individuals. PLOS ONE. 10 (6), 0128780(2015).
- Kiss, B., et al. Retinal Blood Flow during Hyperoxia in Humans Revisited: Concerted Results Using Different Measurement Techniques. Microvascular Research. 64 (1), 75-85 (2002).
- Yezhuvath, U. S., Lewis-Amezcua, K., Varghese, R., Xiao, G., Lu, H. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR in biomedicine. 22 (7), 779-786 (2009).
- Hardarson, S. H., et al. Automatic Retinal Oximetry. Investigative Ophthalmology & Visual Science. 47 (11), 5011(2006).
- Sousa, D. C., Leal, I., Moreira, S., Dionísio, P., Abegão Pinto, L., Marques-Neves, C. Hypoxia challenge test and retinal circulation changes - a study using ocular coherence tomography angiography. Acta Ophthalmologica. 96 (3), 315-319 (2018).
- Slessarev, M., Somogyi, R., Preiss, D., Vesely, A., Sasano, H., Fisher, J. A. Efficiency of oxygen administration: Sequential gas delivery versus "flow into a cone" methods. Critical Care Medicine. 34 (3), 829-834 (2006).
- Gilmore, E. D., Hudson, C., Venkataraman, S. T., Preiss, D., Fisher, J. Comparison of Different Hyperoxic Paradigms to Induce Vasoconstriction: Implications for the Investigation of Retinal Vascular Reactivity. Investigative Ophthalmology & Visual Science. 45 (9), 3207(2004).
- Shahidi, A. M., Patel, S. R., Huang, D., Tan, O., Flanagan, J. G., Hudson, C. Assessment of total retinal blood flow using Doppler Fourier Domain Optical Coherence Tomography during systemic hypercapnia and hypocapnia. Physiological Reports. 2 (7), 12046(2014).
- Maleki, N., et al. The Effect of Hypercarbia and Hyperoxia on the Total Blood Flow to the Retina as Assessed by Magnetic Resonance Imaging. Investigative Ophthalmology & Visual Science. 52 (9), 6867(2011).
- Smit, B., Smulders, Y. M., vander Wouden, J. C., Oudemans-van Straaten, H. M., Spoelstra-de Man, A. M. E. Hemodynamic effects of acute hyperoxia: systematic review and meta-analysis. Critical Care. 22 (1), 45(2018).
- Piccolino, F. P., Cagini, C., Fruttini, D., Nicolò, M., Eandi, C. M., Tito, S. Retinal Vascular Reactivity in Central Serous Chorioretinopathy. Investigative Ophthalmology & Visual Science. 59 (11), 4425(2018).
- Sousa, D. C., et al. A Protocol to Evaluate Retinal Vascular Response Using Optical Coherence Tomography Angiography. Frontiers in Neuroscience. 13, 566(2019).
- Robinson, F., Riva, C. E., Grunwald, J. E., Petrig, B. L., Sinclair, S. H. Retinal Blood Flow Autoregulation in Response to on Acute Increase in Blood Pressure. Investigative Ophthalmology & Visual Science. 27 (5), 5(1986).
- Gherghel, D., Hosking, S. L., Cunliffe, I. A. Abnormal Systemic and Ocular Vascular Response to Temperature Provocation in Primary Open-Angle Glaucoma Patients: A Case for Autonomic Failure. Investigative Ophthalmology & Visual Science. 45 (10), 3546(2004).
- You, Q., et al. Reproducibility of vessel density measurement with Optical Coherence Tomography Angiography in eyes with and without retinopathy. Retina. 37 (8), 1475-1482 (2017).
- Lei, J., et al. Repeatability and Reproducibility of Superficial Macular Retinal Vessel Density Measurements Using Optical Coherence Tomography Angiography En Face Images. JAMA Ophthalmology. 135 (10), 1092(2017).
- Czakó, C., et al. Intrasession and Between-Visit Variability of Retinal Vessel Density Values Measured with OCT Angiography in Diabetic Patients. Scientific Reports. 8 (1), 10598(2018).
- Field, A. S., Laurienti, P. J., Yen, Y. F., Burdette, J. H., Moody, D. M. Dietary Caffeine Consumption and Withdrawal: Confounding Variables in Quantitative Cerebral Perfusion Studies. Radiology. 227 (1), 129-135 (2003).
- Baek, S. U., et al. Diurnal change of retinal vessel density and mean ocular perfusion pressure in patients with open-angle glaucoma. PLOS ONE. 14 (4), 0215684(2019).
- Müller, V. C., Storp, J. J., Kerschke, L., Nelis, P., Eter, N., Alnawaiseh, M. Diurnal variations in flow density measured using optical coherence tomography angiography and the impact of heart rate, mean arterial pressure and intraocular pressure on flow density in primary open-angle glaucoma patients. Acta Ophthalmologica. 97 (6), (2019).
- Sarwar, S., et al. Diurnal variation of choriocapillaris vessel flow density in normal subjects measured using optical coherence tomography angiography. International Journal of Retina and Vitreous. 4 (1), 37(2018).
- Liu, P., De Vis, J. B., Lu, H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. NeuroImage. 187, 104-115 (2019).
- Ting, D. S. W., et al. Optical Coherence Tomographic Angiography in Type 2 Diabetes and Diabetic Retinopathy. JAMA Ophthalmology. 135 (4), 306(2017).
- Spaide, R. F., Fujimoto, J. G., Waheed, N. K., Sadda, S. R., Staurenghi, G. Optical coherence tomography angiography. Progress in retinal and eye research. 64, 1-55 (2018).
- An, D., et al. Quantitative comparisons between optical coherence tomography angiography and matched histology in the human eye. Experimental Eye Research. 170, 13-19 (2018).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved