A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Isolation and Functional Assessment of Human Breast Cancer Stem Cells from Cell and Tissue Samples
In This Article
Summary
This experimental protocol describes the isolation of BCSCs from breast cancer cell and tissue samples as well as the in vitro and in vivo assays that can be used to assess BCSC phenotype and function.
Abstract
Breast cancer stem cells (BCSCs) are cancer cells with inherited or acquired stem cell-like characteristics. Despite their low frequency, they are major contributors to breast cancer initiation, relapse, metastasis and therapy resistance. It is imperative to understand the biology of breast cancer stem cells in order to identify novel therapeutic targets to treat breast cancer. Breast cancer stem cells are isolated and characterized based on expression of unique cell surface markers such as CD44, CD24 and enzymatic activity of aldehyde dehydrogenase (ALDH). These ALDHhighCD44+CD24- cells constitute the BCSC population and can be isolated by fluorescence-activated cell sorting (FACS) for downstream functional studies. Depending on the scientific question, different in vitro and in vivo methods can be used to assess the functional characteristics of BCSCs. Here, we provide a detailed experimental protocol for isolation of human BCSCs from both heterogenous populations of breast cancer cells as well as primary tumor tissue obtained from breast cancer patients. In addition, we highlight downstream in vitro and in vivo functional assays including colony forming assays, mammosphere assays, 3D culture models and tumor xenograft assays that can be used to assess BCSC function.
Introduction
Understanding the cellular and molecular mechanisms of human breast cancer stem cells (BCSCs) is crucial for addressing the challenges encountered in breast cancer treatment. The emergence of the BCSC concept dates back to the early 21st century, where a small population of CD44+CD24-/low breast cancer cells were found to be capable of generating heterogenous tumors in mice1,2. Subsequently, it was observed that human breast cancer cells with high enzymatic activity of aldehyde dehydrogenase (ALDHhigh) also displayed similar stem cell-like properties3. These BCSCs represent a small population of cells capable of self-renewal and differentiation, contributing to the heterogenous nature of bulk tumors1,2,3. Accumulating evidence suggest that alterations in evolutionarily conserved signaling pathways drive BCSC survival and maintenance4,5,6,7,8,9,10,11,12,13,14. In addition, the cell extrinsic microenvironment has been shown to play a pivotal role in dictating different BCSC functions15,16,17. These molecular pathways and the external factors regulating BCSC function contribute to breast cancer relapse, metastasis18 and development of resistance to therapies19,20,21, with the residual existence of BCSCs post-treatment posing a major challenge to the overall survival of breast cancer patients22,23. Pre-clinical evaluation of these factors is therefore very important for identifying BCSC-targeting therapies that could be beneficial for achieving better treatment outcomes and improved overall survival in breast cancer patients.
Several in vitro human breast cancer cell line models and in vivo human xenograft models have been used to characterize BCSCs24,25,26,27,28,29. The ability of cell lines to continuously repopulate after every successive passage makes these an ideal model system to perform omics-based and pharmacogenomic studies. However, cell lines often fail to recapitulate the heterogeneity observed in patient samples. Hence, it is important to complement cell line data with patient-derived samples. Isolation of BCSCs in their purest form is important for enabling detailed characterization of BCSCs. Achieving this purity depends on the selection of phenotypic markers that are specific to BCSCs. Currently, the ALDHhighCD44+CD24- cell phenotype is most commonly used to distinguish and isolate human BCSCs from bulk breast cancer cell populations using fluorescence activated cell sorting (FACS) for maximum purity1,3,26. Furthermore, the properties of isolated BCSCs such as self-renewal, proliferation, and differentiation can be evaluated using in vitro and in vivo techniques.
For example, in vitro colony forming assays can be used to assess the ability of a single cell to self-renew to form a colony of 50 cells or more in presence of different treatment conditions30. Mammosphere assays can also be used to assess the self-renewal potential of breast cancer cells under anchorage-independent conditions. This assay measures the ability of single cells to generate and grow as spheres (mixture of BCSCs and non-BCSCs) at each successive passage in serum-free non-adherent culture conditions31. Additionally, 3-Dimensional (3D) culture models can be used to assess BCSC function, including cell-cell and cell-matrix interactions that closely recapitulate the in vivo microenvironment and allow investigation of the activity of potential BCSC-targeted therapies32. Despite the diverse applications of in vitro models, it is difficult to model the complexity of in vivo conditions using only in vitro assays. This challenge can be overcome by use of mouse xenograft models to evaluate BCSC behavior in vivo. In particular, such models serve as an ideal system for assessing breast cancer metastasis33, investigating interactions with the microenvironment during disease progression34, in vivo imaging35, and for predicting patient-specific toxicity and efficacy of antitumor agents34.
This protocol provides a detailed description for the isolation of human ALDHhighCD44+CD24- BCSCs at maximum purity from bulk populations of heterogenous breast cancer cells. We also provide a detailed description of three in vitro techniques (colony forming assay, mammosphere assay, and 3D culture model) and an in vivo tumor xenograft assay that can be used to assess different functions of BCSCs. These methods would be appropriate for use by investigators interested in isolating and characterizing BCSCs from human breast cancer cell lines or primary-patient derived breast cancer cells and tumor tissue for the purposes of understanding BCSC biology and/or investigating novel BCSC-targeting therapies.
Protocol
Collection of patient-derived surgical or biopsy samples directly from consenting breast cancer patients were carried out under approved human ethics protocol approved by the institutional ethic board. All mice used to generate patient-derived xenograft models were maintained and housed in an institution approved animal facility. The tumor tissue from patient-derived xenograft models using mice were generated as per approved ethics protocol approved by the institutional animal care committee.
1. Preparation of cell lines
- Perform all cell culture and staining procedures under sterile conditions in a biosafety cabinet. Use sterile cell culture dishes/flasks and reagents.
- Maintain human breast cancer cells at 37 °C with 5% CO2 in defined media supplemented with fetal bovine serum (FBS) and necessary growth factors specific to each cell line.
- Maintain mouse NIH3T3 fibroblast cell cultures (for use in colony forming assays) at 37 °C with 5% CO2 in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% FBS.
- For all cultures, replenish the old media every 2-3 days with fresh media. Once the cultures reach 75-80% confluency, subculture into multiple sterile cell culture flasks.
2. Preparation of breast cancer tumor tissue
- Collect the patient-derived surgical or biopsy samples directly from consenting breast cancer patients under a human ethics protocol approved by the institutional ethics board.
- Subsequently, collect and generate tumor tissue from patient-derived xenograft models using mice under an animal ethics protocol approved by the institutional animal care committee.
- Collect all tumor tissues under sterile conditions into a 50 mL sterile conical tube containing 30 mL DMEM:F12 media, keep on ice, and process the samples as described below within 2 h of collection.
3. Generation of single cell suspensions of breast cancer cells
- Aspirate media from the flask containing a monolayer of breast cancer cells that is 60-80% confluent (cell lines of choice). Wash the cells with 1x phosphate buffered saline (PBS). Aspirate PBS and add appropriate cell dissociation solution (e.g., Trypsin:EDTA; just enough to cover the monolayer of cells) and incubate for 5 min at room temperature (recommended) or at 37 °C.
- Add 5 mL of culture media to neutralize the activity of cell dissociation solution.
- Transfer the resulting dissociated cell solution to a 50 mL conical tube and centrifuge at 1000 x g for 5 min.
- Discard supernatant and resuspended the cell pellet in 5 mL of 1x PBS. Count the cells using a hemocytometer and a microscope.
NOTE: Observe for cell clumping in the hemocytometer. Repeat cell dissociation step if single cell suspension has not formed. - After cell counting, re-centrifuge the cell suspension at 1000 x g for 5 min, discard supernatant, and resuspend the cell pellet in ALDH substrate buffer at a concentration of 1 x 106 cells/mL.
4. Generation of single cell suspension from tissue samples
- Mince the tumor tissue with surgical blades using a crisscross technique to obtain smaller pieces of approximately 1 mm in size. Transfer the tissue pieces into a fresh 50 mL conical tube containing 10 mL dissociation buffer (1X Collagenase in DMEM:F12). Seal the conical tube with parafilm and incubate at 37 °C in a shaker incubator for 40 min.
NOTE: If there is not a shaker incubator, place the tube in a 37 °C water bath and mix the tube by vortexing every 5-10 min. - Pellet the digested tissue by centrifuging sample at 530 x g for 5 min. Discard the supernatant and add 5 mL of trypsin. Pipette up and down using 1 mL pipette (set to 750 µL mark) to disrupt the pellet and incubate in a 37 °C water bath for 5 min. After incubation, pipette up and down vigorously to release single cells.
- Top up the total volume in the tube to 25 mL with DMEM:F12 media and centrifuge at 1000 x g for 5 min. Discard the supernatant and resuspend the pellet in 1 mL of dispase-DNase solution. Incubate in a 37 °C water bath for 5 min.
- Top up the total volume in the tube to 10 mL with PBS. Mix by pipetting up and down, pass the resulting cell suspension through a 40 µm cell strainer attached to a fresh 50 mL conical tube. Centrifuge at 1000 x g for 5 min.
- Discard supernatant and resuspend the cell pellet in 5 mL of 1x PBS. Count the cells and complete preparation of the cell suspension as described in steps 3.4 and 3.5.
5. Isolation of breast cancer stem cells (BCSCs)
- Label flow tubes for the unstained control, single cell staining controls (DEAB control, ALDH, CD44-PE, CD24-PE-Cy7, 7AAD), the negative control tube (stained with DEAB, CD44-PE, CD24-PE-Cy7 and 7AAD), fluorescent minus one (FMO) control and the ‘sort’ tube (stained with ALDH, CD44, CD24 and 7AAD).
- Transfer 500 μL (0.5 x 106 cells) of the cell suspensions from step 3.5 or step 4.5 to each tube that is labelled cells only, CD44, CD24 and 7AAD. Place the tubes on ice until use.
- Transfer 2 mL of sample (2 x 106 cells) to respective ‘ALDH’ tube. Add 5 μL of DEAB to the ‘DEAB control’ and ‘negative control’ tubes and cap it tightly. Add 10 μL of ALDH substrate to the ‘ALDH’ tube, mix well by vortexing, and immediately transfer 500 μL to corresponding ‘DEAB control’ and ‘negative control’ tube. Recap the ‘DEAB control’, ‘negative control’ and ‘ALDH tubes’ and incubate at 37 °C for 30-60 min (do not exceed 60 min).
NOTE: The optimal incubation time may require optimization depending on the cell line. Always protect the ALDH substrate and the tubes containing stained cells from light. - Following incubation, centrifuge all samples for 5 min at 250 x g. Resuspend the cells in 500 μL of ALDH substrate buffer. Add manufacturer-recommended or user-optimized concentration of anti-CD44-PE and anti-CD24-PE-Cy7 antibody cocktail and incubate at 4 °C for 30 min. Add anti-CD44-PE and anti-CD24-PE-Cy7 antibodies to respective ‘CD44’ and ‘CD24’ labelled tubes.
- Following incubation, centrifuge all samples at 250 x g for 5 min. Resuspend the cells in 500 μL of ALDH substrate buffer. Incubate the ‘negative control’ tube, ‘Sort tube’ and the ‘7ADD’ tube with 7AAD (suggested concentration: 0.25 µg/1 x 106 cells) for 10 min on ice.
NOTE: The ALDH activity is detected in the green fluorescent channel, therefore a fluorochrome with a different compatible emission spectrum should be used. Where spectral overlap is observed during multi-parameter flow cytometry, single color controls and FMO control should be used as a guide to allow compensation between fluorochromes to minimize the spill over of fluorescent signal into other channels. - Set up the analysis protocol on the FACS instrument in preparation for sample analysis. Create scatter plots (forward vs side scatter, forward scatter vs fluorescent channels).
- Using the unstained control, adjust the photomultiplier to separate debris from whole cell population and adjust the fluorescent voltage to move the whole cell population around the first log scale (101). Using the DEAB control, move the whole cell population within the second log scale (102) by adjusting the green fluorescent voltage channel.
- Analyze all the single staining controls first (ALDH, CD44-PE, CD24-PE-Cy7) and 7AAD and FMO control, adjusting the voltage to separate stained from unstained cells and to minimize the spillage of fluorescent signals into other channels.
- Gate on the positive population for each single stained cell sample. Using the negative control tube, gate for viability (7AAD negative), ALDHlow and ALDHhigh cell populations (representative gating strategy shown in Figure 1B).
- Analyze multiparameter stained samples of interest to isolate BCSCs. Using the viable ALDHlow and ALDHhigh gates, select for CD44+CD24- (BCSC) and CD44-CD24+ (non-BCSC) cell population respectively (Figure 1B).
- Collect viable BCSCs and non-BCSCs in collection media in sterile collection tubes (populations from two representative cell lines shown in Figure 2A&B). Use sorted cells for downstream in vitro and in vivo assays as described below.
NOTE: In addition to in vitro and in vivo assays described below, BCSCs can be validated by measuring the expression of pluripotent markers such as SOX2, OCT4 and NANOG via standard immunoblotting techniques.
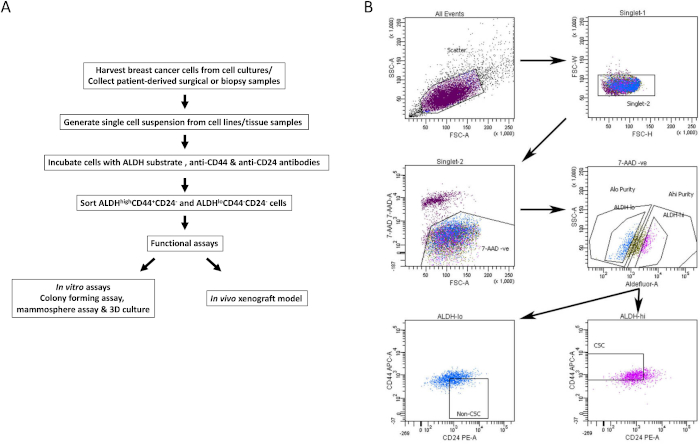
Figure 1: FACS gating strategy for isolation of BCSCs from breast cancer cell lines and tissue samples. (A) Flowchart describing the procedure of BCSC isolation. (B) Representative FACS plots showing the sort strategy used to isolate viable BCSCs and non-BCSCs from a heterogenous pool of cells. MDA-MB-231 human breast cancer cells are concurrently labeled with 7-AAD, CD44-APC, CD24-PE and the ALDH substrate. Cell subsets were isolated using a four-color protocol on a FACS machine. Cells are selected based on expected light scatter, then for singlets, and viability based on 7-AAD exclusion. Cells are then analyzed for ALDH activity and the top 20% most positive are selected as the ALDHhigh population, while the bottom 20% of cells with the lowest ALDH activity were deemed to be ALDHlow. Finally, 50% of the ALDHlow cells are further selected based on a CD44low/-CD24+ phenotype, and 50% of the ALDHhigh cells are selected based on CD44+CD24- phenotype. This figure has been adapted from Chu et al.17. Please click here to view a larger version of this figure.
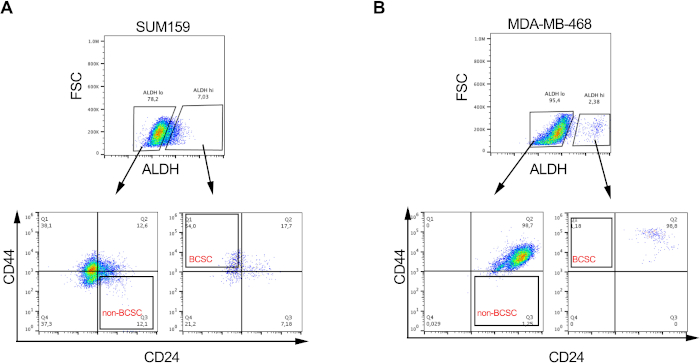
Figure 2: BCSCs proportions are variable in different breast cancer cell lines. Representative image showing the differential proportion of BCSCs and non-BCSCs in (A) SUM159 and (B) MDA-MD-468 triple negative breast cancer cell lines following labelling and sorting as described in Figure 1. Please click here to view a larger version of this figure.
6. Colony forming assay
- Resuspend the cells of interest (sorted cells from step 5.11 or unsorted cells from steps 3.5 or 4.5) in complete media.
- Label three flow tubes for 1 x 102, 2 x 102 and 5 x 102 cells. Add 2 mL of complete media and transfer the appropriate cell number (sorted from step 5.11 or unsorted cells from steps 3.5 or 4.5) in respective tubes. Mix the cell solutions thoroughly by pipetting it up and down 5 times.
- Plate the cells in a 6-well plate and distribute the cell suspension by gently swirling the plates to obtain uniform distribution of cells.
- Incubate the plates in a 37 °C, 5% CO2 incubator until colonies appear (where colonies = ≥50 cells per colony). Carefully replenish media twice a week without disturbing colony formation.
- Aspirate media and wash once with 1 mL PBS. Add 0.5 mL of 0.05% crystal violet solution into each well and incubate the plate for 30 minutes. Remove excess crystal violet stain by washing with 2 mL of water. Repeat the washing step until background staining has been removed.
- Using a microscope at 4x and 10x magnification, count and record the total number of colonies generated (representative images shown in Figure 3A).
- Calculate the frequency of colony formation as follows: Frequency (%) = (# of colonies formed/number of cells seeded) x 100. For example, if 25 colonies are generated from 1 x 102 cells, then the Frequency of colony formation is, Frequency = (25/100) x100 = 25%.
- Alternatively, replace steps 6.1 to 6.4 with an alternate method involving co-culture with fibroblasts, which provide a microenvironmental support for BCSCs through production of necessary growth and survival factors.
- Pre-coat cell 60 mm culture dishes with type I bovine collagen (1 in 30 dilution of 3 mg/mL collagen). Allow collagen to polymerize for 30 min in a 37 °C incubator. Aspirate the unpolymerized collagen and wash the plate twice with 1x PBS. Cover the collagen-coated plate with 1 mL of PBS and set it aside at room temperature until use.
- Label three flow tubes for 1 x 103, 5 x 103 and 1 x 104 cells. Add 4 mL of colony forming assay media and transfer the appropriate number of cells (sorted from step 5.11 or unsorted cells from steps 3.5 or 4.5) into the respective tubes. Add irradiated mouse NIH3T3 fibroblasts (4 x 104 cells/mL of media). Mix cell solutions thoroughly by pipetting it up and down 5 times.
- Aspirate the PBS from the collagen-coated culture dish from step 6.1 and plate the cell mixture onto each of the cell culture plates as described in step 6.3.
- Incubate the plates in a 37 °C, 5% CO2 incubator and leave them undisturbed for 7-10 days or until colonies form, without replenishing the media. Count and record the total number of colonies generated as described in steps 6.6 and 6.7.
7. Mammosphere assay
- Resuspend the cells of interest (sorted cells from step 5.11 or unsorted cells from steps 3.5 or 4.5) in complete mammosphere media and plate cells at a seeding density of 5 x 102 cells/cm2 area in a 96 well ultra-low attachment cell culture plate.
NOTE: Cell seeding density should be optimized for different cell lines. - Incubate the culture plates for 5-10 days in a 37 °C incubator with 5% CO2. Carefully replenish media twice a week without disturbing mammosphere formation.
- After incubation, count the number of mammospheres generated in each well using a microscope; where mammospheres are defined as breast cancer cell clusters greater than 100 μm in diameter (representative images shown in Figure 3B).
- Calculate the mammosphere formation efficiency (MFE) as follows: MFE (%) = (number of mammospheres per well)/ (number of cells seeded per well) x 100 (i.e., if 5 mammospheres are generated by 1 x 102 cells in a well, then MFE = (5/100) x 100 = 5%).
- To subculture mammospheres, carefully transfer the media containing mammospheres content into a fresh 50 mL conical tube and centrifuge media at 1000 x g for 5 min. Carefully remove the supernatant, resuspend the cell pellet in 500 μL of trypsin, and incubate for 5 min at room temperature.
- Discard the supernatant and resuspend the pellet in 1 mL of complete mammosphere media. Count the cells using a hemocytometer and re-plate the cells in an ultra-low attachment cell culture plate as described in step 7.1.
NOTE: In addition to sub-culturing, the mammosphere-derived cells can be also analyzed further by FACS to assess BCSC phenotype and/or obtain pure populations of BCSCs for other downstream assays. - To determine the number of mammosphere-initiating cells contained within your cell populations, use an alternate method involving sphere limiting dilution analysis (SLDA). Plate cells in serial dilutions of high to low cell numbers in a 96 well ultra-low attachment cell culture plate, with the highest dilution resulting in less than one cell per well.
- Incubate the culture plate for 10-14 days in a 37 °C incubator with 5% CO2 and leave them undisturbed to avoid cell aggregation.
- After incubation, count the number of mammospheres generated in each well using a microscope; where mammospheres are defined as breast cancer cell clusters greater than 100 μm in diameter. Calculate the sphere-initiating frequency and significance using Extreme Limiting Dilution Analysis (ELDA) online software (http://bioinf.wehi.edu.au/software/elda/).
8. 3D culture model
- Depending on the experimental question, use basement membrane extract (BME) with or without growth factors (reduced). In order to evaluate the effect of individual growth factor on cancer cells, use growth factor reduced BME. It also helps in minimizing the non-specific effects of endogenous growth factors present in BME.
NOTE: BME solidifies above 10 °C. Always keep BME on ice even during the thawing step. - Carefully add 50 μL of BME per well in a 96-well plate without creating air bubbles and allow it to polymerize at 37 °C for 1 h. After 10 min of incubation, add 100 μL PBS to avoid drying of the gel layer.
- Resuspend the sorted cells from step 5.11 or unsorted cells from steps 3.5 or 4.5 at a concentration of 5 x 103 to 5 x 104/200 μL in 3D culture media.
- Once the BME has polymerized, remove PBS, add 200 µL of cell suspension to each well and incubate in 37 °C incubator with 5% CO2. Add PBS to the surrounding wells to avoid evaporation of the media.
NOTE: The optimal number of cells for plating should be determined prior to setting of the experiment. Depending on the experimental question, BCSCs can be cultured alone or with other cells types (fibroblasts/endothelial/immune cells etc.). - Add fresh media to the culture plates twice weekly. Maintain cultures for 10-14 days prior to analyzing the formation of organoids (representative images shown in Figure 3C).
- For sub-culturing, carefully aspirate the media and add 200 μL of dispase to each well containing cells. Incubate the plate in a 37 °C incubator for 1 h. Halfway through the incubation period (30 min), take out the plate, gently pipette the dispase solution up and down 5 times, and place back in the incubator for a further 30 min.
- After 1 h, transfer the dissociated cell solution to a flow tube. Wash the well with 1x PBS containing 2% FBS (fPBS) and transfer it to the flow tube. Centrifuge the tube at 1000 x g for 5 min. Carefully aspirate the supernatant and add 500 μL of trypsin, incubate at 37 °C for 5 min. Inactivate trypsin by adding equal amount of fPBS and centrifuge at 1000 x g for 5 min.
- Discard the supernatant and resuspend the pellet in 1 mL of 3D culture media. Count the cells and re-plate required number of cells in the BME as in steps 8.2 to 8.4.
NOTE: Multiple wells can be pooled to further analyze or sort the cell population of interest.
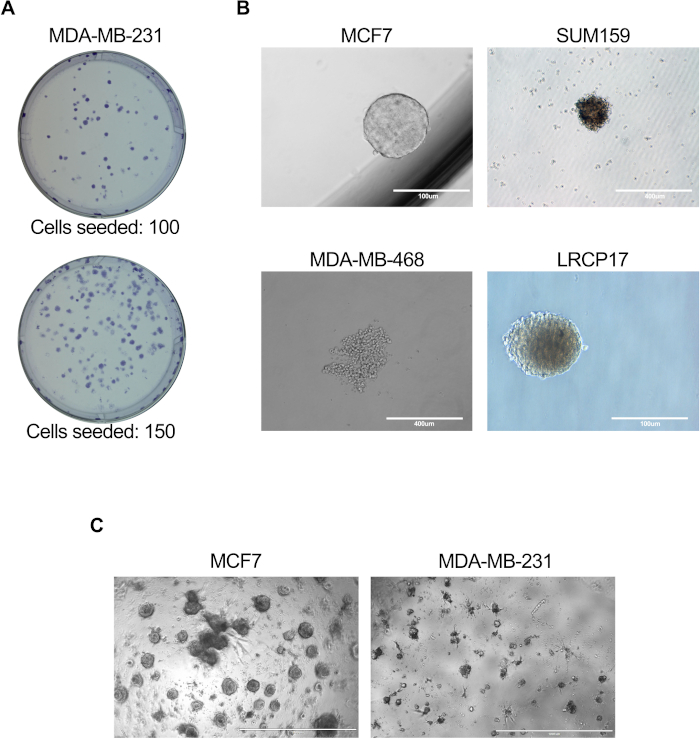
Figure 3: In vitro assays to assess BCSC cell function. In vitro assays were performed as described in protocol sections 6.1 to 6.5 (A), 7.1 to 7.4 (B), or 81. to 8.4 + 8.6 (C). (A) Representative image showing the colonies generated by MDA-MB-231 human breast cancer cells; (B) Representative images showing mammosphere formation by MCF7, SUM159, or MDA-MB-468 human cell lines as well as patient-derived LRCP17 breast cancer cells. (C) Representative images showing the 3D structures formed by MCF7 and MDA-MB-231 breast cancer cells in 3D cultures models. Please click here to view a larger version of this figure.
NOTE: Perform animal experiments under an animal ethics protocol approved by the institutional animal care committee.
9. In vivo xenograft model
- In order to determine the tumor initiation capacity of breast cancer stem cells, prepare cells (sorted population from step 5.7 or unsorted populations from steps 3.5 or 4.5) using a limiting dilution approach. Serially dilute cells in PBS using between 1 and 5 different dilution groups, with doses as low as 0.01-0.2 x 102 cells/100 µL and as high as 1 x 106 cells/100 µL.
NOTE: Unsorted/whole population cells can be used as a control. The number of dilution groups used will depend on the desired scientific outcome (e.g. if only testing tumorgenicity then 1 group at a higher cell number may be used, whereas when calculating tumor-initiating capacity, it is optimal to test 5 limiting dilution doses). - To generate xenograft models from human breast cancer cells, use immunocompromised female mice (athymic nude [nu/nu], nonobese diabetic/severe combined immunodeficient [NOD/SCID] or NOD/SCID IL2γ [NGS] strains).
NOTE: Although a minimum of 4 animals per group can be used, 8-12 animals per group is recommended to obtain robust results particularly for limiting dilution analysis. - Perform standard mammary fat pad (MFP) injections using 100 µL/mouse of each cell preparation, under sterile conditions in a biosafety cabinet.
NOTE: For optimal breast tumor growth and spontaneous metastasis to distant organs, the thoracic MFP is recommended. Alternatively, the inguinal MFP can also be used. - Post-injection, monitor the mice on a daily basis for general health and tumor growth at the site of injection. Upon detection of a palpable tumor, begin measuring the tumor size by calipers in two perpendicular dimensions and record weekly until endpoint.
NOTE: The experimental end point is determined based on the regulations laid out the institutional animal ethics protocol; typically, endpoint by euthanasia is usually required once tumor volumes reach 1500 mm3. For BCSC populations and/or higher cell doses (e.g. >1 x 104 cells), this endpoint will likely be reached within 4-8 weeks of MFP injection. For very low cell doses and/or non-BCSC cell populations, tumor growth should be allowed to progress for up to 8 months post-injection. - From these measurements, calculate the tumor volume using the following formula: Volume in mm3 = 0.52 x (width)2 x length. If using a limiting dilution approach, calculate tumor-initiating capacity and significance using ELDA online software (http://bioinf.wehi.edu.au/software/elda/).
- Alternatively, to humanely extend the endpoint, surgically remove primary tumors and continue to monitor mice for health and/or development of spontaneous metastasis in distant organs. Use resected tumor tissue for the generation of serial xenotransplants.
- At endpoint, harvest tissue from primary tumors and distant organs (lymph nodes, lung, liver, brain, bone) and carry out histopathological and/or immunohistochemical analysis or dissociated the tumor tissue and use in the in vitro assays described in sections 6-8.
Results
The described protocol allows isolation of human BCSCs from a heterogenous population of breast cancer cells, either from cell lines or from dissociated tumor tissue. For any given cell line or tissue sample, it is crucial to generate a uniform single cell suspension to isolate BCSCs at maximum purity as contaminating non-BCSC populations could result in variable cellular responses, especially if the study aim is to evaluate the efficacy of therapeutic agents targeting BCSCs. Application of a stringent sorting strategy w...
Discussion
Breast cancer metastasis and resistance to therapy have become major cause of mortality in women worldwide. The existence of a sub-population of breast cancer stem cells (BCSCs) contributes to enhanced metastasis26,43,44,45,46 and therapy resistance21,47,48. Therefore...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank members of our laboratory for their helpful discussions and support. Our research on breast cancer stem cells and the tumor microenvironment is funded by grants from the Canadian Cancer Research Society Research Institute and the U.S. Army Department of Defense Breast Cancer Program (Grant # BC160912). V.B. is supported by a Western Postdoctoral Fellowship (Western University), and both A.L.A. and V.B. are supported by the Breast Cancer Society of Canada. C.L. is supported by a Vanier Canada Graduate Scholarship from the Government of Canada.
Materials
| Name | Company | Catalog Number | Comments |
| 7-Aminoactinomycin D (7AAD) | BD | 51-68981E | suggested: 0.25 µg/1x106 cells |
| Acetone | Fisher | A18-1 | |
| Aldehyde dehydrogenase (ALDH) substrate | Stemcell Technologies | 1700 | Sold commerically as part of the ALDEFLOUR Assay kit; follow manufacturer's instructions for ALDH substrate preparation |
| Basement membrane extract (BME) | Corning | 354234 | Sold under the commercial name Matrigel |
| Cell culture plates: 6 well | Corning | 877218 | |
| Cell culture plates: 60mm | Corning | 353002 | |
| Cell culture plates: 96-well ultra low attachment | Corning | 3474 | |
| Cell strainer: 40 micron | BD | 352340 | |
| Collagen | Stemcell Technologies | 7001 | Prepare 1:30 dilution of 3 mg/mL collagen in PBS |
| Collagenase | Sigma | 11088807001 | 1x |
| Conical tubes: 50 mL | Fisher scientific | 05-539-7 | |
| Crystal violet | Sigma | C6158 | Use 0.05% crystal violet solution in water for staining |
| Dispase | Stemcell Technologies | 7913 | 5U/mL |
| DMEM:F12 | Gibco | 11330-032 | 1x, With L-glutamine and 15 mM HEPES |
| DNAse | Sigma | D5052 | 0.1 mg/mL final concentration |
| FBS | Avantor Seradigm Lifescience | 97068-085 | |
| Flow tubes: 5ml | BD | 352063 | Polypropylene round-bottom tubes |
| Methanol | Fisher | 84124 | |
| mouse anti-Human CD24 antibody | BD | 561646 | R-phycoerythrin and Cyanine dye conjugated Clone: ML5 |
| mouse anti-Human CD44 antibody | BD | 555479 | R-phycoerythrin conjugated, Clone: G44-26 |
| N,N-diethylaminobenzaldehyde (DEAB) | Stemcell Technologies | 1700 | Sold commerically as part of the ALDEFLOUR Assay kit; follow manufacturer's instructions DEAB preparation |
| PBS | Wisent Inc | 311-425-CL | 1x, Without calcium and magnesium |
| Trypsin-EDTA | Gibco | 25200-056 | |
| Mammosphere Media Composition | |||
| B27 | Gibco | 17504-44 | 1x |
| bFGF | Sigma | F2006 | 10 ng/mL |
| BSA | Bioshop | ALB003 | 04% |
| DMEM:F12 | Gibco | 11330-032 | 1x, With L-glutamine and 15 mM HEPES |
| EGF | Sigma | E9644 | 20 ng/mL |
| Insulin | Sigma | 16634 | 5 µg/mL |
| 3D Organoid Media Composition | |||
| A8301 | Tocris | 2939 | 500 nM |
| B27 | Gibco | 17504-44 | 1x |
| DMEM:F12 | Gibco | 11330-032 | 1x, With L-glutamine and 15 mM HEPES |
| EGF | Sigma | E9644 | 5 ng/mL |
| FGF10 | Peprotech | 100-26 | 20 ng/mL |
| FGF7 | Peprotech | 100-19 | 5 ng/mL |
| GlutaMax | Invitrogen | 35050-061 | 1x |
| HEPES | Gibco | 15630-080 | 10 mM |
| N-acetylcysteine | Sigma | A9165 | 1.25 mM |
| Neuregulin β1 | Peprotech | 100-03 | 5 nM |
| Nicotinamide | Sigma | N0636 | 5 mM |
| Noggin | Peprotech | 120-10C | 100 ng/mL |
| R-spondin3 | R&D | 3500 | 250 ng/mL |
| SB202190 | Sigma | S7067 | 500 nM |
| Y-27632 | Tocris | 1254 | 5 µM |
References
- Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 100 (7), 3983-3988 (2003).
- Shipitsin, M., et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 11 (3), 259-273 (2007).
- Ginestier, C., et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 1 (5), 555-567 (2007).
- Sulaiman, A., et al. Dual inhibition of Wnt and Yes-associated protein signaling retards the growth of triple-negative breast cancer in both mesenchymal and epithelial states. Molecular Oncology. 12 (4), 423-440 (2018).
- Debeb, B. G., et al. Histone deacetylase inhibitors stimulate dedifferentiation of human breast cancer cells through WNT/β-catenin signaling. Stem Cells. 30 (11), 2366-2377 (2012).
- Klutzny, S., et al. PDE5 inhibition eliminates cancer stem cells via induction of PKA signaling. Cell Death & Disease. 9 (2), 192 (2018).
- DiMeo, T. A., et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Research. 69 (13), 5364-5373 (2009).
- Liu, C. C., Prior, J., Piwnica-Worms, D., Bu, G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proceedings of the National Academy of Sciences of the United States of America. 107 (11), 5136-5141 (2010).
- Miller-Kleinhenz, J., et al. Dual-targeting Wnt and uPA receptors using peptide conjugated ultra-small nanoparticle drug carriers inhibited cancer stem-cell phenotype in chemo-resistant breast cancer. Biomaterials. 152, 47-62 (2018).
- Mamaeva, V., et al. Inhibiting Notch Activity in Breast Cancer Stem Cells by Glucose Functionalized Nanoparticles Carrying γ-secretase Inhibitors. Molecular Therapy. 24 (5), 926-936 (2016).
- Ithimakin, S., et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Research. 73 (5), 1635-1646 (2013).
- Koike, Y., et al. Anti-cell growth and anti-cancer stem cell activities of the non-canonical hedgehog inhibitor GANT61 in triple-negative breast cancer cells. Breast Cancer. 24 (5), 683-693 (2017).
- Sun, Y., et al. Estrogen promotes stemness and invasiveness of ER-positive breast cancer cells through Gli1 activation. Molecular Cancer. 13, 137 (2014).
- Colavito, S. A., Zou, M. R., Yan, Q., Nguyen, D. X., Stern, D. F. Significance of glioma-associated oncogene homolog 1 (GLI1) expression in claudin-low breast cancer and crosstalk with the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway. Breast Cancer Research. 16 (5), 444 (2014).
- Bhat, V., Allan, A. L., Raouf, A. Role of the Microenvironment in Regulating Normal and Cancer Stem Cell Activity: Implications for Breast Cancer Progression and Therapy Response. Cancers. 11 (9), (2019).
- Pio, G. M., Xia, Y., Piaseczny, M. M., Chu, J. E., Allan, A. L. Soluble bone-derived osteopontin promotes migration and stem-like behavior of breast cancer cells. PloS One. 12 (5), 0177640 (2017).
- Chu, J. E., et al. Lung-derived factors mediate breast cancer cell migration through CD44 receptor-ligand interactions in a novel ex vivo system for analysis of organ-specific soluble proteins. Neoplasia. 16 (2), 180-191 (2014).
- McGowan, P. M., et al. Notch1 inhibition alters the CD44hi/CD24lo population and reduces the formation of brain metastases from breast cancer. Molecular Cancer Research. 9 (7), 834-844 (2011).
- Mao, J., et al. ShRNA targeting Notch1 sensitizes breast cancer stem cell to paclitaxel. International Journal of Biochemistry and Cell Biology. 45 (6), 1064-1073 (2013).
- Duru, N., et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clinical Cancer Research. 18 (24), 6634-6647 (2012).
- Croker, A. K., Allan, A. L. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44+ human breast cancer cells. Breast Cancer Research and Treatment. 133 (1), 75-87 (2012).
- Creighton, C. J., et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proceedings of the National Academy of Sciences of the United States of America. 106 (33), 13820-13825 (2009).
- Calcagno, A. M., et al. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. Journal of the National Cancer Institute. 102 (21), 1637-1652 (2010).
- Feng, Y., et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 5 (2), 77-106 (2018).
- Samanta, D., Gilkes, D. M., Chaturvedi, P., Xiang, L., Semenza, G. L. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America. 111 (50), 5429-5438 (2014).
- Croker, A. K., et al. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. Journal of Cellular and Molecular Medicine. 13 (8), 2236-2252 (2009).
- Morel, A. P., et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PloS One. 3 (8), 2888 (2008).
- Muntimadugu, E., Kumar, R., Saladi, S., Rafeeqi, T. A., Khan, W. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf B Biointerfaces. 143, 532-546 (2016).
- Liu, S., et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2 (1), 78-91 (2014).
- Munshi, A., Hobbs, M., Meyn, R. E. Clonogenic cell survival assay. Methods in Molecular Medicine. 110, 21-28 (2005).
- Shaw, F. L., et al. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. Journal of Mammary Gland Biology and Neoplasia. 17 (2), 111-117 (2012).
- Shin, C. S., Kwak, B., Han, B., Park, K. Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug. Molecular Pharmaceutics. 10 (6), 2167-2175 (2013).
- Khanna, C., Hunter, K. Modeling metastasis in vivo. Carcinogenesis. 26 (3), 513-523 (2005).
- Cheon, D. J., Orsulic, S. Mouse models of cancer. Annual Review of Pathology. 6, 95-119 (2011).
- Lyons, S. K. Advances in imaging mouse tumour models in vivo. Journal of Pathology. 205 (2), 194-205 (2005).
- Margaryan, N. V., et al. The Stem Cell Phenotype of Aggressive Breast Cancer Cells. Cancers. 11 (3), (2019).
- Ma, F., et al. Enriched CD44(+)/CD24(-) population drives the aggressive phenotypes presented in triple-negative breast cancer (TNBC). Cancer Letters. 353 (2), 153-159 (2014).
- Chatterjee, S., et al. Paracrine Crosstalk between Fibroblasts and ER(+) Breast Cancer Cells Creates an IL1β-Enriched Niche that Promotes Tumor Growth. iScience. 19, 388-401 (2019).
- Phan-Lai, V., et al. Three-dimensional scaffolds to evaluate tumor associated fibroblast-mediated suppression of breast tumor specific T cells. Biomacromolecules. 14 (5), 1330-1337 (2013).
- O'Brien, C. A., Kreso, A., Jamieson, C. H. Cancer stem cells and self-renewal. Clinical Cancer Research. 16 (12), 3113-3120 (2010).
- Hu, Y., Smyth, G. K. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. Journal of Immunological Methods. 347 (1-2), 70-78 (2009).
- Stewart, J. M., et al. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proceedings of the National Academy of Sciences of the United States of America. 108 (16), 6468-6473 (2011).
- Abraham, B. K., et al. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clinical Cancer Research. 11 (3), 1154-1159 (2005).
- Balic, M., et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clinical Cancer Research. 12 (19), 5615-5621 (2006).
- Charafe-Jauffret, E., et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clinical Cancer Research. 16 (1), 45-55 (2010).
- Marcato, P., et al. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 29 (1), 32-45 (2011).
- Lacerda, L., Pusztai, L., Woodward, W. A. The role of tumor initiating cells in drug resistance of breast cancer: Implications for future therapeutic approaches. Drug Resist Updat. 13 (4-5), 99-108 (2010).
- Liu, S., Wicha, M. S. Targeting breast cancer stem cells. Journal of Clinical Oncology. 28 (25), 4006-4012 (2010).
- D'Angelo, R. C., et al. Notch reporter activity in breast cancer cell lines identifies a subset of cells with stem cell activity. Molecular Cancer Therapeutics. 14 (3), 779-787 (2015).
- Neve, R. M., et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 10 (6), 515-527 (2006).
- Forozan, F., et al. Comparative genomic hybridization analysis of 38 breast cancer cell lines: a basis for interpreting complementary DNA microarray data. Cancer Research. 60 (16), 4519-4525 (2000).
- Lanier, L. L. Just the FACS. Journal of Immunology. 193 (5), 2043-2044 (2014).
- Ibrahim, S. F., van den Engh, G. Flow cytometry and cell sorting. Advances in Biochemical Engineering/Biotechnology. 106, 19-39 (2007).
- Shapiro, H. M. Flow Cytometry: The Glass Is Half Full. Methods in Molecular Biology. 1678, 1-10 (2018).
- Tsuji, K., et al. Effects of Different Cell-Detaching Methods on the Viability and Cell Surface Antigen Expression of Synovial Mesenchymal Stem Cells. Cell Transplantation. 26 (6), 1089-1102 (2017).
- Sun, C., et al. Immunomagnetic separation of tumor initiating cells by screening two surface markers. Scientific Reports. 7, 40632 (2017).
- Rodríguez, C. E., et al. Breast cancer stem cells are involved in Trastuzumab resistance through the HER2 modulation in 3D culture. Journal of Cellular Biochemistry. 119 (2), 1381-1391 (2018).
- Kim, D. W., Cho, J. Y. NQO1 is Required for β-Lapachone-Mediated Downregulation of Breast-Cancer Stem-Cell Activity. International Journal of Molecular Sciences. 19 (12), (2018).
- Xu, L. Z., et al. p62/SQSTM1 enhances breast cancer stem-like properties by stabilizing MYC mRNA. Oncogene. 36 (3), 304-317 (2017).
- Huang, X., et al. Breast cancer stem cell selectivity of synthetic nanomolar-active salinomycin analogs. BMC Cancer. 16, 145 (2016).
- Liu, T. J., et al. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 32 (5), 544-553 (2013).
- Ponti, D., et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Research. 65 (13), 5506-5511 (2005).
- Velasco-Velázquez, M. A., Popov, V. M., Lisanti, M. P., Pestell, R. G. The role of breast cancer stem cells in metastasis and therapeutic implications. American Journal of Pathology. 179 (1), 2-11 (2011).
- Palomeras, S., Ruiz-Martínez, S., Puig, T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Molecules. 23 (9), (2018).
- McClements, L., et al. Targeting treatment-resistant breast cancer stem cells with FKBPL and its peptide derivative, AD-01, via the CD44 pathway. Clinical Cancer Research. 19 (14), 3881-3893 (2013).
- Berger, D. P., Henss, H., Winterhalter, B. R., Fiebig, H. H. The clonogenic assay with human tumor xenografts: evaluation, predictive value and application for drug screening. Annals of Oncology. 1 (5), 333-341 (1990).
- Tian, J., et al. Dasatinib sensitises triple negative breast cancer cells to chemotherapy by targeting breast cancer stem cells. British Journal of Cancer. 119 (12), 1495-1507 (2018).
- Samoszuk, M., Tan, J., Chorn, G. Clonogenic growth of human breast cancer cells co-cultured in direct contact with serum-activated fibroblasts. Breast Cancer Research. 7 (3), 274-283 (2005).
- Linnemann, J. R., et al. Quantification of regenerative potential in primary human mammary epithelial cells. Development. 142 (18), 3239-3251 (2015).
- Xu, Y., Hu, Y. D., Zhou, J., Zhang, M. H. Establishing a lung cancer stem cell culture using autologous intratumoral fibroblasts as feeder cells. Cell Biology International. 35 (5), 509-517 (2011).
- Palmieri, C., et al. Fibroblast growth factor 7, secreted by breast fibroblasts, is an interleukin-1beta-induced paracrine growth factor for human breast cells. Journal of Endocrinology. 177 (1), 65-81 (2003).
- Bourguignon, L. Y., Peyrollier, K., Xia, W., Gilad, E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. Journal of Biological Chemistry. 283 (25), 17635-17651 (2008).
- Yin, X., et al. Engineering Stem Cell Organoids. Cell Stem Cell. 18 (1), 25-38 (2016).
- Sachs, N., et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. 172 (1-2), 373-386 (2018).
- Kim, M., et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nature Communications. 10 (1), 3991 (2019).
- Okano, M., et al. Orthotopic Implantation Achieves Better Engraftment and Faster Growth Than Subcutaneous Implantation in Breast Cancer Patient-Derived Xenografts. Journal of Mammary Gland Biology and Neoplasia. 25 (1), 27-36 (2020).
- Zhang, Y., et al. Establishment of a murine breast tumor model by subcutaneous or orthotopic implantation. Oncology Letters. 15 (5), 6233-6240 (2018).
- Zhang, W., et al. Comparative Study of Subcutaneous and Orthotopic Mouse Models of Prostate Cancer: Vascular Perfusion, Vasculature Density, Hypoxic Burden and BB2r-Targeting Efficacy. Scientific Reports. 9 (1), 11117 (2019).
- Kim, R., Emi, M., Tanabe, K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 121 (1), 1-14 (2007).
- Rosato, R. R., et al. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Research. 20 (1), 108 (2018).
- Choi, Y., et al. Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Experimental and Molecular Medicine. 50 (8), 99 (2018).
- Meraz, I. M., et al. An Improved Patient-Derived Xenograft Humanized Mouse Model for Evaluation of Lung Cancer Immune Responses. Cancer Immunol Res. 7 (8), 1267-1279 (2019).
- Wege, A. K. Humanized Mouse Models for the Preclinical Assessment of Cancer Immunotherapy. Biodrugs. 32 (3), 245-266 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved