A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Preparation of Expanded Chitin Foams and their Use in the Removal of Aqueous Copper
In This Article
Summary
This study describes a method to expand chitin into a foam by chemical techniques that require no specialized equipment.
Abstract
Chitin is an underexploited, naturally abundant, mechanically robust, and chemically resistant biopolymer. These qualities are desirable in an adsorbent, but chitin lacks the necessary specific surface area, and its modification involves specialized techniques and equipment. Herein is described a novel chemical procedure for expanding chitin flakes, derived from shrimp shell waste, into foams with higher surface area. The process relies on the evolution of H2 gas from the reaction of water with NaH trapped in a chitin gel. The preparation method requires no specialized equipment. Powder X-ray diffraction and N2-physisorption indicate that the crystallite size decreases from 6.6 nm to 4.4 nm and the specific surface area increases from 12.6 ± 2.1 m2/g to 73.9 ± 0.2 m2/g. However, infrared spectroscopy and thermogravimetric analysis indicate that the process does not change the chemical identity of the chitin. The specific Cu adsorption capacity of the expanded chitin increases in proportion to specific surface area from 13.8 ± 2.9 mg/g to 73.1 ± 2.0 mg/g. However, the Cu adsorption capacity as a surface density remains relatively constant at an average of 10.1 ± 0.8 atom/nm2, which again suggests no change in the chemical identity of the chitin. This method offers the means to transform chitin into a higher surface area material without sacrificing its desirable properties. Although the chitin foam is described here as an adsorbent, it can be envisioned as a catalyst support, thermal insulator, and structural material.
Introduction
Chitin is a mechanically robust and chemically inert biopolymer, second only to cellulose in natural abundance1. It is the major component in the exoskeleton of arthropods and in the cell walls of fungi and yeast2. Chitin is similar to cellulose, but with one hydroxyl group of each monomer replaced with an acetyl amine group (Figure 1A,B). This difference increases the strength of hydrogen bonding between adjacent polymer chains and gives chitin its characteristic structural resilience and chemical inertness2,3. Due to its properties and abundance, chitin has attracted significant industrial and academic interest. It has been studied as a scaffold for tissue growth4,5,6, as a component in composite materials7,8,9,10,11, and as a support for adsorbents and catalysts11,12,13,14. Its chemical stability, in particular, makes chitin attractive for adsorption applications that involve conditions inhospitable to common adsorbents14. In addition, the abundance of amine groups make chitin an effective adsorbent for metal ions15. However, the protonation of the amine groups under acidic conditions reduces the metal adsorption capacity of chitin16. A successful strategy is to introduce adsorption sites more resistant to protonation17,18. Instead, herein is described a simple method to increase the specific surface area and, therefore, the number of adsorption sites in chitin.
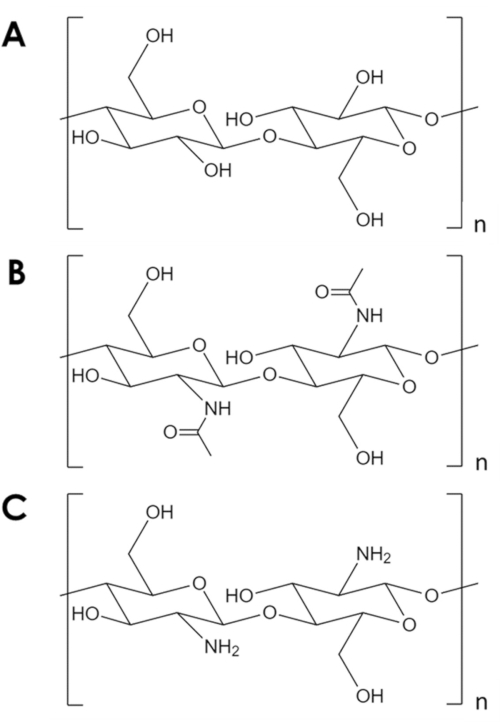
Figure 1. Chemical structure. (A) cellulose, (B) chitin, (C) chitosan. Please click here to view a larger version of this figure.
In spite of its many potential uses, chitin is underutilized. Chitin processing is challenging due to its low solubility in most solvents. A key limitation to its use in catalysis and adsorption is its low specific surface area. While typical carbon and metal oxide supports have specific surface areas in the order 102-103 m2/g, commercial chitin flakes have surface areas in the order of 10 m2/g19,20,21. Methods to expand chitin into foams exist, but they invariably rely on high temperature and pressure, strong acids and bases, or specialized equipment that represent a significant entry barrier5,21,22,23,24,25. In addition, these methods tend to deacetylate chitin to form chitosan (Figure 1C)-a more soluble and reactive biopolymer5,25,26.
Herein, a method is described to expand chitin into solid foams, increase its specific surface area and adsorption capacity, and maintain its chemical integrity. The method relies on the rapid evolution of gas from within a chitin gel and requires no specialized equipment. The increased adsorption capacity of the expanded chitin is demonstrated with aqueous Cu2+-a common contaminant in the local groundwater26.
| Unit | Neat Flake | Baked Foam | Lyophilised Foam | |
| Crystallinity | % | 88 | 74 | 58 |
| Crystal size | nm | 6.5 | 4.4 | 4.4 |
| Surface Area | m2/g | 12.6 ± 2.1 | 43.1 ± 0.2 | 73.9 ± 0.2 |
| Cu Uptake | mg/g | 13.8 ± 2.9 | 48.6 ± 1.9 | 73.1 ± 2.0 |
| Cu Uptake | atom/nm2 | 10.5 ± 2.8 | 10.7 ± 0.4 | 9.4 ± 0.3 |
Table 1. Summary of material properties. Chitin foams have lower crystallinity and crystal size relative to neat chitin flakes. However, the specific surface area and Cu uptake of the chitin foams are proportionally higher than that of the neat chitin flakes.
Protocol
1. Preparation of expanded chitin
- Prepare a 250 mL solution of 5 wt% LiCl in dimethylacetamide (DMAc)
CAUTION: The solvent DMAc is a combustible irritant that may damage fertility and cause birth defects. Handle DMAc in a fume hood using chemical resistant gloves and goggles to avoid contact with skin and eyes.- Add 15 g of LiCl and 285 g (268 mL) of DMAc into a 500 mL Erlenmeyer flask with, then place a 50 mm Polytetrafluoroethylene (PTFE)-lined magnetic stir bar.
- Cap the flask with a rubber septum and place it on a heating stir plate. Place a temperature probe through the septum into the mixture. Stir the mixture at 400 rpm and 80 °C until all LiCl is dissolved (~ 4 h)
- Dissolve 1.0 g of oven-dried chitin flakes in the LiCl/DMAc solution to form a sol-gel
- Dry at least 1.2 g of chitin flakes in an oven at 80°C for 24 h.
- Add 1.0 g of oven-dried chitin flakes and 250 mL of 5 wt% LiCl/DMAc solution into a 500 mL round bottom flask. Place a 50 mm PTFE-lined magnetic stir bar.
- Cap the flask with a rubber septum and place it on a stirring heat block. Pierce the septum with a needle and leave it to allow the flask to vent. Heat the block to 80 °C and stir the mixture at 400 rpm until all the chitin is dissolved (24-48 h).
- Allow the resultant chitin sol-gel to cool down to room temperature slowly while continuing to stir (~ 1 h).
- Once at room temperature, place the flask containing the chitin sol-gel in an ice bath and continue stirring until its temperature stabilizes (~ 20 min).
- Prepare a 100 mL slurry of NaH in DMAc.
CAUTION: NaH in contact with water releases flammable gases which may ignite spontaneously. To limit contact with moist air, NaH is stored in mineral oil which must be washed off before use. Handle with caution in a fume hood using chemical resistant gloves and goggles.- Remove approximately 1 g of NaH from its mineral oil storage and wash three times with 10 mL of hexanes.
- Add 100 mL of DMAC into a 250 mL Erlenmeyer flask, then add 0.82 g of the washed NaH and place a PTFE-lined magnetic stir bar.
- Swirl the mixture to produce a NaH/DMAc slurry.
NOTE: NaH will not completely dissolve.
- Form the chitin gel by adding all the NaH/DMAc slurry to the chitin sol-gel.
- Uncap the cooled sol-gel and add all the NaH slurry while stirring vigorously. Replace the cap and continue to stir the mixture at 400 rpm for 72 h or until a gel forms in the flask.
- Form the chitin foam by adding water to the chitin gel.
- After the formation of the gel, uncap the flask and add 100 mL of Deionized (DI) water.
NOTE: It is critical to perform this step in a fume hood as the process will evolve H2 gas.
- After the formation of the gel, uncap the flask and add 100 mL of Deionized (DI) water.
- Isolate, and wash the chitin foam in water and methanol to remove DMAc and salts.
- Remove the expanded chitin foam from the flask and place in a crystallization dish or beaker sufficiently large to hold it and 1000 mL of DI water.
NOTE: The chitin foam will not come out in one piece and may have to be broken up. - Rinse the isolated gel three times with 500 mL of DI water. Soak the gel in 1000 mL of DI water for 24 h, then in 500 mL of methanol for 24 h, and finally in 1000 mL of DI water for 24 h again.
- Remove the expanded chitin foam from the water wash and allow to air dry for 24-48 h.
- Remove the expanded chitin foam from the flask and place in a crystallization dish or beaker sufficiently large to hold it and 1000 mL of DI water.
- Dry the washed chitin gel to form a solid foam and then grind to a powder.
- Dry the gel in the oven at 85 °C for 48 h under ambient air, or in a lyophilizer at -43 °C and 0.024 mbar for 48 h.
- Using a mortar and pestle, grind the dry chitin foam into a fine powder.
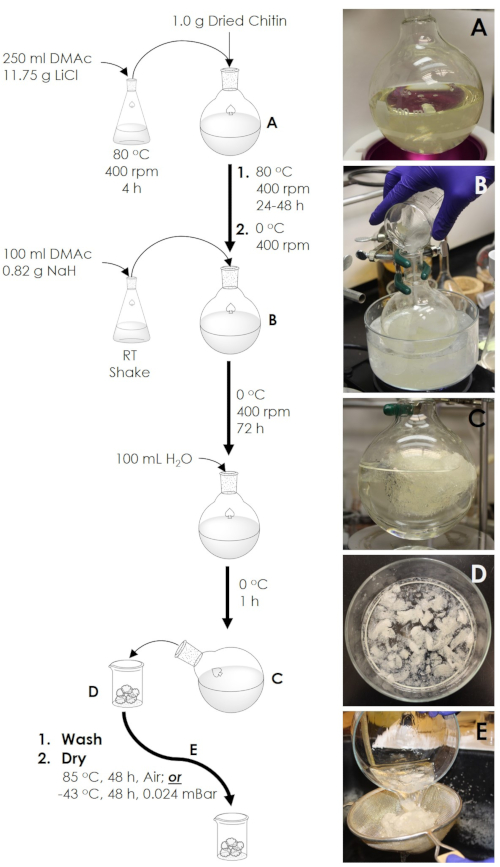
Figure 2. Preparation of expanded chitin foam. (A) The initial chitin in LiCl/DMAc solution. (B) The addition of the NaH/DMAc slurry. (C) The chitin foam after addition of water. (D) The chitin foam as extracted from the reaction flask. (E) The chitin foam during washing with water. Please click here to view a larger version of this figure.
2. Development of the adsorption isotherms
- Prepare 500 mL stock solutions of aq. Cu2+ (MW 63.5 g/mol) at concentrations 50 mg/L, 100 mg/L, 200 mg/L, 300 mg/L, 400 mg/L, and 450 mg/L. To do this, add 90 mg, 180 mg, 360 mg, 540 mg, 720 mg, and 810 mg of Cu(NO3)2· 2.5 H2O (MW 232.6 g/mol) to six containers, respectively. Add 500 mL of 18 MΩ water, cap the container, and shake to dissolve the solids.
- Add 50 mg of chitin to 100 mL of each stock solution, adjust the pH to 7, and allow to equilibrate for 48 h.
- Transfer 100 mL of each stock solution to a 100 mL container so the headspace is minimal. Add 50 mg of ground chitin to each container and then cap them.
- Place containers on an orbital shaker and shake at 60 rpm for 30 min. Then take containers off the orbital shaker and adjust the pH to 7 using NH4HCO3 or HNO3.
- Replace containers back on the orbital shaker and shake at 60 rpm and at a constant temperature for 48 h. Maintain the laboratory at 18 ± 2 °C throughout.
- Measure the Cu concentration of the initial stock solutions and of those to which chitin was added. Use the colorimetric bicinchoninate method, a colorimeter, and pre-measured reagent packets27.
- Remove containers from the orbital shaker, allow the mixtures to settle for a minimum of 30 min, and then take a 1 mL aliquot with a syringe fitted with a 0.3 µm glass microfiber filter.
- Transfer the aliquot to a 250 mL container and dilute to 100 mL with 18 MΩ water.
NOTE: This step is necessary due to the low ceiling of detection of Cu (5 mg/L) by the bicinchoninate method using the colorimeter. - Transfer 10 mL of the diluted sample to a cuvette. Place the cuvette in the colorimeter and zero the instrument.
- Add one packet of premeasured Cu reagent (bicinchoninate method) to the diluted sample in the cuvette and wait 45 s for the chelation reaction to complete. Allow the solution to become purple. The intensity of the color formed is proportional to the Cu concentration.
- Place the cuvette back in the colorimeter and measure the Cu concentration of the diluted sample. Multiply the concentration of the diluted sample by 100 to obtain that of the original sample.
- Extract the maximum Cu uptake from the adsorption isotherm data.
- Calculate the uptake of each sample for each equilibrium Cu concentration using the equation28:

- Plot the adsorption uptake versus equilibrium concentration of the samples to produce a standard Cu adsorption isotherm.
- Plot the ratio of equilibrium concentration to uptake versus the equilibrium concentration to produce the linearized Cu adsorption isotherm.
NOTE: The plot should be linear, and the inverse of the slope represents the maximum Cu uptake.
- Calculate the uptake of each sample for each equilibrium Cu concentration using the equation28:
Results
Expanded chitin shows the same morphology regardless of the drying method. Figure 3 shows images of neat chitin flakes (Figure 3A1), oven-dried expanded chitin (Figure 3B1), and lyophilized expanded chitin (Figure 3C3). While the neat flakes have the appearance of coarse sand, the expanded chitin foam has the appearance of a kernel of popped corn. Scanning electron micrographs show a similar ch...
Discussion
The proposed method for chitin foam fabrication allows for the production of such foams without the need for specialized equipment or techniques. Production of the chitin foam relies on the suspension of sodium hydride within a chitin sol-gel. Contact with water from the atmosphere induces gelling of the chitin matrix and evolution of hydrogen gas by decomposition of the sodium hydride. Therefore, the critical steps of the preparation are (1) formation of the sol-gel, (2) introduction of the sodium hydride in anhydrous c...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The research was sponsored by the Combat Capabilities Development Command Army Research Laboratory (Cooperative Agreement Number W911NF-15-2-0020). Any opinions, findings and conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Army Research Lab.
We thank the Center for Advanced Materials Processing (CAMP) at Montana Technological University for the use of some of the specialized equipment required in this study. We also thank Gary Wyss, Nancy Oyer, Rick LaDouceur, John Kirtley, and Katherine Zodrow for the technical assistance and helpful discussions.
Materials
| Name | Company | Catalog Number | Comments |
| Ammonium bicarbonate | Sigma-Aldrich | 9830 | NH4HCO3, ≥99.5 % |
| Chitin | Sigma-Aldrich | C7170 | Pandalus borealis, practical grade |
| Colorimeter | Hanna Instruments | HI83399-01 | Photometer for wastewater analysis |
| Copper High Range Checker | Hanna Instruments | HI702 | Bicinchoninate colorimetric titration |
| Copper nitrate hydrate | Sigma-Aldrich | 223395 | Cu(NO3)2 · 2.5 H2O, 98 % |
| Dimethylacetamide (DMAc) | Sigma-Aldrich | 271012 | Anhydrous, 99.8 % |
| IR Spectrophotometer | Thermo Nicolet | Nexus 670 | Fitted with an ATR cell |
| Lithium chloride | Sigma-Aldrich | 310468 | LiCl, ≥99 % |
| N2 Physisorption Apparatus | Micromeritics | Tristar II | |
| Nitric acid | BDH | BDH7208-1 | HNO3, 0.1 N |
| Scanning electron microscope | Zeiss LEO | 1430 VP | 15 kV, secondary electron detector, 29-31 mm working distance |
| Sodium hydride | Sigma-Aldrich | 223441 | NaH, packed in mineral oil, 90 % |
| Thermogravimetric analyzer | TA Instruments | Q500 | 100 ml/min N2, 10 °C/min to 800 °C |
| Water Purification System | Millipore | Milli-Q | Type A water (18 MΩ) |
| X-Ray Diffractometer | Rigaku | Ultima IV | Cu K-α radiation, 8.04 keV |
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress in Polymer Science. 31 (7), 603-632 (2006).
- Percot, A., Viton, C., Domard, A. Optimization of chitin extraction from shrimp shells. Biomacromolecules. 4 (1), 12-18 (2003).
- Austin, P. R. Chitin solvents and solubility parameters. Chitin, Chitosan, and Related Enzymes. , 227-237 (1984).
- Deepthi, S., Venkatesan, J., Kim, S. K., Bumgardner, J. D., Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. International Journal of Biological Macromolecules. 93, 1338-1353 (2016).
- Tao, F., et al. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydrate Polymers. 230, 115658 (2020).
- Zhao, L., et al. Regulation of the morphological and physical properties of a soft tissue scaffold by manipulating DD and DS of O-carboxymethyl chitin. ACS Applied Bio Materials. 3 (9), 6187-6195 (2020).
- Duan, Y., Freyburger, A., Kunz, W., Zollfrank, C. Cellulose and chitin composite materials from an ionic liquid and a green co-solvent. Carbohydrate Polymers. 192, 159-165 (2018).
- Kadokawa, J., Takegawa, A., Mine, S., Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydrate Polymers. 84 (4), 1408-1412 (2011).
- Chen, Z., Wang, J., Qi, H. J., Wang, T., Naguib, H. E. Green and sustainable layered chitin-vitrimer composite with enhanced modulus, reprocessability, and smart actuator function. ACS Sustainable Chemistry and Engineering. 8 (40), 15168-15178 (2020).
- Zhang, Z., Lucia, L. A. Chitin-clay composite gels with enhanced thermal stability prepared in a green and facile approach. Journal of Materials Science. 56 (4), 3600-3611 (2021).
- Ahmed, M. J., Hameed, B. H., Hummadi, E. H. Review on recent progress in chitosan/chitin-carbonaceous material composites for the adsorption of water pollutants. Carbohydrate Polymers. 247, 116690 (2020).
- Matsuoka, A., et al. Hydration of nitriles to amides by a chitin-supported ruthenium catalyst. RSC Advances. 5 (16), 12152-12160 (2015).
- Wang, Y., Li, Y., Liu, S., Li, B. Fabrication of chitin microspheres and their multipurpose application as catalyst support and adsorbent. Carbohydrate Polymers. 120, 53-59 (2015).
- Anastopoulos, I., Bhatnagar, A., Bikiaris, D., Kyzas, G. Chitin Adsorbents for Toxic Metals: A Review. International Journal of Molecular Sciences. 18 (1), 114 (2017).
- Habiba, U., Afifi, A. M., Salleh, A., Ang, B. C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. Journal of Hazardous Materials. 322, 182-194 (2017).
- Kim, U. J., et al. Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohydrate Polymers. 163, 34-42 (2017).
- He, Y., et al. Fabrication of PVA nanofibers grafted with octaamino-POSS and their application in heavy metal adsorption. Journal of Polymers and the Environment. , (2020).
- Tian, H., et al. Electrospinning of polyvinyl alcohol into crosslinked nanofibers: An approach to fabricate functional adsorbent for heavy metals. Journal of Hazardous Materials. 378, (2019).
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Applied Catalysis A: General. 315, 1-17 (2006).
- Dotto, G. L., Cunha, J. M., Calgaro, C. O., Tanabe, E. H., Bertuol, D. A. Surface modification of chitin using ultrasound-assisted and supercritical CO2 technologies for cobalt adsorption. Journal of Hazardous Materials. 295, 29-36 (2015).
- Phongying, S., Aiba, S., Chirachanchai, S. Direct chitosan nanoscaffold formation via chitin whiskers. Polymer. 48 (1), 393-400 (2007).
- Tan, T. S., Chin, H. Y., Tsai, M. L., Liu, C. L. Structural alterations, pore generation, and deacetylation of α- and β-chitin submitted to steam explosion. Carbohydrate Polymers. 122, 321-328 (2015).
- Chang, F. S., Chin, H. Y., Tsai, M. L. Preparation of chitin with puffing pretreatment. Research on Chemical Intermediates. 44 (8), 4939-4955 (2018).
- Goodrich, J. D., Winter, W. T. α-Chitin Nanocrystals prepared from shrimp shells and their specific surface area measurement. Biomacromolecules. 8 (1), 252-257 (2007).
- Rolandi, M., Felts, J. . Naturally sourced chitin foam. , (2020).
- McDermott, S., Hailer, M. K., Lead, J. R. Meconium identifies high levels of metals in newborns from a mining community in the U.S. Science of the Total Environment. 707, 135528 (2020).
- Hach Handbook of Water Analysis. Copper, Bicinchoninate Method, Method 8506. Hach Handbook of Water Analysis. , (1979).
- Crittenden, J. C., Trusell, R. R., Hand, D. R., Howe, K. J., Tchbanoglous, G. Adsorption. MWH's Water Treatment. , 1117 (2012).
- Focher, B., Beltrame, P. L., Naggi, A., Torri, G. Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohydrate Polymers. 12 (4), 405-418 (1990).
- Scherrer, P. Determination of the size and the internal structure of colloidal particles by means of X-rays. News from the Society of Sciences in Göttingen, Mathematical- Physical Class. 2, 98-100 (1918).
- Brunauer, S., Emmett, P. H., Teller, E. Adsorption of gases in multimolecular layers. Journal of the American Chemical Society. 60 (2), 309-319 (1938).
- Sing, K. S. W. Adsorption methods for the characterization of porous materials. Advances in Colloid and Interface Science. 76-77, 3-11 (1998).
- Rouquerol, J., Llewellyn, P., Rouquerol, F. Is the bet equation applicable to microporous adsorbents. Studies in Surface Science and Catalysis. 160, 49-56 (2007).
- Vorokh, A. S. Scherrer formula: estimation of error in determining small nanoparticle size. Nanosystems: Physics, Chemistry, Mathematics. , 364-369 (2018).
- Labidi, A., Salaberria, A. M., Fernandes, S. C. M., Labidi, J., Abderrabba, M. Adsorption of copper on chitin-based materials: Kinetic and thermodynamic studies. Journal of the Taiwan Institute of Chemical Engineers. 65, 140-148 (2016).
- Tian, M., Zhao, T. Q., Chin, P. L., Liu, B. S., Cheung, A. S. -. C. Methane and propane co-conversion study over zinc, molybdenum and gallium modified HZSM-5 catalysts using time-of-flight mass-spectrometry. Chemical Physics Letters. 592, 36-40 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved