A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Ganglioside Extraction, Purification and Profiling
In This Article
Summary
Gangliosides are sialic acid-bearing glycosphingolipids that are particularly abundant in the brain. Their amphipathic nature requires organic/aqueous extraction and purification techniques to ensure optimal recovery and accurate analyses. This article provides overviews of analytic and preparative scale ganglioside extraction, purification, and thin layer chromatography analysis.
Abstract
Gangliosides are glycosphingolipids that contain one or more sialic acid residues. They are found on all vertebrate cells and tissues but are especially abundant in the brain. Expressed primarily on the outer leaflet of the plasma membranes of cells, they modulate the activities of cell surface proteins via lateral association, act as receptors in cell-cell interactions and are targets for pathogens and toxins. Genetic dysregulation of ganglioside biosynthesis in humans results in severe congenital nervous system disorders. Because of their amphipathic nature, extraction, purification, and analysis of gangliosides require techniques that have been optimized by many investigators in the 80 years since their discovery. Here, we describe bench-level methods for the extraction, purification, and preliminary qualitative and quantitative analyses of major gangliosides from tissues and cells that can be completed in a few hours. We also describe methods for larger scale isolation and purification of major ganglioside species from brain. Together, these methods provide analytical and preparative scale access to this class of bioactive molecules.
Introduction
Gangliosides are defined as glycosphingolipids bearing one or more sialic acid residues1. They are expressed primarily at the cell surface with their hydrophobic ceramide lipid moiety embedded in the outer leaflet of the plasma membrane and their hydrophilic glycans extending into the extracellular space2. Although distributed widely in vertebrate cells and tissues, they are particularly abundant in the vertebrate brain3, where they were first discovered and named4.
The structures of ganglioside glycans vary and are the basis for their nomenclature (Figure 1). Ganglioside glycans are comprised of a neutral sugar core bearing different numbers and distributions of sialic acids. The smallest ganglioside, GM4, has only two sugars (sialic acid bound to galactose)5. Larger naturally occurring gangliosides may contain well over a dozen total sugars6 or up to seven sialic acids on a single neutral core7. Their ceramide lipid moieties also vary, having different sphingosine lengths and a variety of fatty acid amides. In the vertebrate brain four ganglioside species, GM1, GD1a, GD1b, and GT1b predominate. Ganglioside expression is developmentally regulated, tissue specific, and cell type specific.

Figure 1: Major brain gangliosides and their biosynthetic precursors. Structures are shown using Symbol Nomenclature for Glycans11. Please click here to view a larger version of this figure.
Gangliosides function at the molecular level by engaging and modulating proteins in their own membranes (cis regulation) or by engaging glycan binding proteins in the extracellular milieu, including bacterial toxins and lectins on other cells (trans recognition)3. Specific binding of gangliosides to regulatory proteins and/or self-association with other molecules into lipid rafts results in changes in cell behavior that impact nervous system structure and function, cancer progression, metabolism, inflammation, neuronal proteinopathies, and infectious diseases8. Because of their diverse cellular roles, methods for their isolation and analysis can provide enhanced insights into the regulation of physiological and pathological processes. Here, validated methods for rapid small-scale extraction and analysis, and preparative scale isolation of gangliosides from brain are provided. Opportunities and challenges for application to other tissues are discussed.
Protocol
Tissue collection was performed under conditions authorized by the Johns Hopkins Animal Care and Use Committee.
1. Small scale ganglioside extraction and partial purification
CAUTION: Use appropriate ventilation when working with volatile and toxic solvents. Avoid plastic throughout; solvents will extract chemical components from many plastics that interfere with subsequent analyses. Polytetrafluoroethylene (PTFE) is an exception; PTFE-lined closures should be used to cap glass storage vials.
- Extraction
- Weigh a single fresh or thawed mouse brain (or sagittal half-brain, ~ 0.2-0.5 g) and place in a Potter-Elvehjem homogenizer prechilled in a bucket of ice.
NOTE: Previously frozen brains can be used after thawing at 0-4 °C. - Add 4.1 mL per g tissue wet weight of water and homogenize with 10 strokes.
NOTE: Accurate solvent ratios are key to optimal extraction and partition, the goal is chloroform-methanol-aqueous (4:8:3) assuming brain tissue is 80% aqueous. - Add 13 mL per g tissue wet weight of methanol, shift to ambient temperature (22 °C) and mix.
NOTE: The solution will appear cloudy. Addition of methanol at this step, without chloroform, optimizes protein precipitation. All subsequent steps are at ambient temperature (22 °C). - Transfer to a thick-walled glass screw-capped tube with a PTFE-lined screw cap at ambient temperature (22 °C) and mix thoroughly. Add 6.5 mL per g tissue wet weight of chloroform, cap, and mix thoroughly. Centrifuge at 450 x g for 15 min. Transfer the clear supernatant to a fresh screw-capped tube and measure the volume, "recovered extract volume".
- Weigh a single fresh or thawed mouse brain (or sagittal half-brain, ~ 0.2-0.5 g) and place in a Potter-Elvehjem homogenizer prechilled in a bucket of ice.
- Partition
- Add 0.173x "recovered extract volume" of water to the clear supernatant, cap, vortex vigorously, and centrifuge as described in step 1.1.4.
NOTE: The goal is to have chloroform-methanol-aqueous in the ratio 4:8:5.6. The mixture will be cloudy and resolve into two phases: an upper aqueous-rich phase and a lower chloroform-rich phase at ~ 4:1 ratio. Wait for 60 min or centrifuge at 450 x g for 15 min for complete phase separation. - Transfer the upper phase, which contains the gangliosides, into a fresh glass tube with a PTFE-lined screw cap.
- Add 0.173x "recovered extract volume" of water to the clear supernatant, cap, vortex vigorously, and centrifuge as described in step 1.1.4.
- Reverse phase cartridge chromatography
- Using a 5 mL glass syringe, wash a tC18 solid phase extraction cartridge (400 mg) with 3 mL of methanol, then 3 mL of chloroform-methanol-water (2:43:55). Load the upper phase from step 1.2.2 onto the tC18 cartridge using the same glass syringe, collect the flow-through and reload it onto the column to optimize adsorption.
- Using the glass syringe, wash the cartridge with 3 mL of chloroform-methanol-water (2:43:55) then 3 mL of methanol-water (1:1).
- Elute the gangliosides with 3 mL of methanol into a fresh screw-capped tube. Evaporate to dryness under a gentle stream of nitrogen at ≤ 45 °C. Dissolve in methanol at 1 mL per g of original tissue wet weight.
2. Large scale ganglioside extraction and purification
CAUTION: When working with volatile solvents, use explosion resistant blenders. Do not use plastics except PTFE. Tetrahydrofuran, chloroform, and ethyl ether are toxic volatile organic compounds. Work in a fume hood with protective gloves and safety goggles.
- Extraction
- Thaw frozen bovine brain at 4 °C for several hours. Dissect the grey matter from meninges and white matter.
NOTE: The following procedure is described for 100 ± 20 g of isolated brain grey matter and is scalable. - Place 100 g of brain grey matter in a blender and add 1 mL per g brain wet weight of chilled 10 mM potassium phosphate buffer pH 6.8. Homogenize on low for 20 s. Add 8 mL tetrahydrofuran per g brain wet weight and homogenize on low for 10 s. Decant into glass centrifuge bottles and centrifuge at 5,000 x g for 15 min at ambient temperature (22 °C).
- Collect the supernatant, measure its volume, and transfer to a glass separatory funnel. Add 0.3 mL of ethyl ether per mL of the supernatant. Shake vigorously, then allow to sit undisturbed for 30 min during which two phases, an upper ether phase and a lower aqueous phase, separate. Collect the lower phase, which contains the gangliosides, into a glass bottle with a PTFE-lined cap.
- To the upper (ether) phase remaining in the separatory funnel, add 0.1 mL water per mL of original supernatant (step 2.1.3). Shake vigorously, allow phases to separate, collect the lower (aqueous) phase and combine with the previous lower phase. Evaporate the combined lower phases to a dry powder and weigh.
- Thaw frozen bovine brain at 4 °C for several hours. Dissect the grey matter from meninges and white matter.
- Saponification
- Add 10 mL of 100 mM aqueous NaOH per g powder in a sealed tube. Mix and incubate at 37 °C for 3 h. Allow to cool and adjust to pH 4.5 by dropwise addition of 100 mM aqueous HCl. Measure the volume and transfer to a glass separatory funnel.
NOTE: Sialic acids are acid labile; avoid acidification below pH 4.5. - Based on the aqueous volume, add 2.67 volumes of methanol, mix gently, then add 1.33 volumes of chloroform to create a single-phase solution of chloroform-methanol-aqueous (4:8:3). Mix well.
- Based on the original aqueous volume, add 2.6 volumes of water to bring the mixture to chloroform-methanol-aqueous to a ratio of 4:8:5.6. Shake vigorously, then allow to sit undisturbed to separate two phases, a polar upper phase containing the gangliosides and a nonpolar lower phase. Collect the upper phase in a glass bottle with a PTFE-lined cap.
NOTE: Non-sialylated lipids will not appear on thin layer chromatography (TLC) plates stained using resorcinol but will appear when using a p-anisaldehyde stain. The purpose of this saponification is to remove the O-acetylated compounds such as phospholipids.
- Add 10 mL of 100 mM aqueous NaOH per g powder in a sealed tube. Mix and incubate at 37 °C for 3 h. Allow to cool and adjust to pH 4.5 by dropwise addition of 100 mM aqueous HCl. Measure the volume and transfer to a glass separatory funnel.
- Reverse phase chromatography
- Pre-wash a large scale (10 g) tC18 solid phase extraction cartridge by passing 50 mL of each of the following three solvents through the column using vacuum or pressure (<1 min each wash): methanol, methanol-water (1:1), then chloroform-methanol-water (2:43:55). Load the upper phase from step 2.2.3 onto the column by vacuum or pressure, collect the flow through, reload, and collect the flow through.
- Wash the column with 30 mL of chloroform-methanol-water (2:43:55), then 30 mL methanol-water (1:1), then elute the gangliosides with 50 mL methanol, and again with 10 mL methanol, collecting each wash and each elution separately. Using TLC (below) confirm that ganglioside is absent from the flow through and washes and eluted in the first elution (50 mL methanol). Evaporate the eluted gangliosides to a dry powder and weigh.
NOTE: The purpose of tC18 chromatography is to separate gangliosides from both less and more polar contaminants. Mixed brain ganglioside yield after saponification is ~ 120 mg per g dry brain extract (step 2.1.4). Appearance of gangliosides by TLC in the flow through or washes indicates the solid phase extraction column was saturated. After methanol elution, the column may be further eluted with chloroform-methanol (1:1) to capture less polar lipids.
- HPLC purification of individual gangliosides
- Prepare HPLC Solvent A: acetonitrile-5 mM aqueous sodium phosphate buffer pH 5.6 (83:17) and Solvent B: acetonitrile-20 mM sodium phosphate buffer, pH 5.6 (1:1). Degas both solvents for 5 min.
- Pre-equilibrate an HPLC column (20 x 250 mm column packed with amine bonded (NH2) silica spheres, 5 µm diameter, 100 Å pore size) with 100% Solvent A for 20 min at 5 mL/min. Set a UV HPLC column effluent detector to 215 nm.
- Dissolve the ganglioside powder from the reverse phase eluate in water at 5 mg/mL. Inject 0.5 mL of the ganglioside mixture onto the HPLC and run the solvent gradient (Table 1) at 5 mL/min, collecting fractions. Gangliosides will appear as A215 peaks with retention times (major brain gangliosides) of 25-70 min: GM1 ≈ 28 min; GD1a ≈ 38 min; GD1b ≈ 46 min; GT1b ≈ 65 min. Re-equilibrate 20 min with Solvent A after each run. Analyze fractions by thin-layer chromatography.
| Time (min) | %A | %B |
| 0 | 100 | 0 |
| 7 | 100 | 0 |
| 12 | 63 | 37 |
| 82 | 54 | 46 |
| 82.01 | 0 | 100 |
| 92 | 0 | 100 |
Table 1: Solvent gradient for HPLC.
3. Thin layer chromatography (TLC) analysis of gangliosides
CAUTION: Chloroform is a toxic volatile organic compound. Work in a fume hood with protective gloves and safety goggles.
- Running solvent and TLC plate preparation
- Prepare a running solvent of chloroform-methanol-aqueous 0.25% KCl (60:35:8 by volume). Pour into a 10 cm x 10 cm glass TLC chamber with a stainless-steel cover so that the solvent depth is ~ 0.5 cm. Cover and allow to equilibrate in an area free of air currents for >10 min.
NOTE:To isolate the TLC chamber from air currents, an acrylic 5-sided box can be constructed or purchased (Figure 2). Do not use solvent-saturated filter paper inside the chamber. - Place a 10 cm x 10 cm or 5 cm x 10 cm silica gel coated glass-backed high performance TLC plate in a drying oven at 125 °C for 10 min. Allow to cool. Use a dulled #2 pencil to draw 5-mm spotting lines with 2-mm separations along a line 1 cm above bottom of the plate and at least 1 cm from either side (Figure 3). Avoid disturbing the silica layer while marking.
- Prepare a standard mix of pure gangliosides in methanol containing 100 µM GM1, 50 µM each of GD1a and GD1b and 33 µM of GT1b.
NOTE: This mixture contains 100 pmol of ganglioside sialic acid per µL for each of the four gangliosides, a quantity that provides a strong colorimetric signal by resorcinol staining, which is sialic acid dependent.
- Prepare a running solvent of chloroform-methanol-aqueous 0.25% KCl (60:35:8 by volume). Pour into a 10 cm x 10 cm glass TLC chamber with a stainless-steel cover so that the solvent depth is ~ 0.5 cm. Cover and allow to equilibrate in an area free of air currents for >10 min.
- Ganglioside resolution
- Wash a 10-µL Hamilton syringe with a beveled needle with methanol. Draw 1 µL methanol into a glass syringe to fill the needle dead volume and then 1 µL of sample or standard. Spot the sample evenly onto the 5-mm premarked lines until <1 µL of solvent (methanol) remains in the syringe. Allow the plate to dry at ambient temperature (22 °C) after all samples are spotted.
NOTE: Wash the syringe with methanol between sample loading. An unheated air blower set at low can be used to accelerate drying. - Place the spotted and dried plate into the preequilibrated TLC chamber with the bottom edge immersed in the running solvent and cover and protect from air currents (Figure 2). Allow the running solvent to advance up the plate by capillary action until the solvent front reaches within 1 cm of the top of the plate. Remove and mark the solvent front at the edge of the plate with a pencil. Allow the solvents to evaporate completely either undisturbed or under mild air flow.
- Wash a 10-µL Hamilton syringe with a beveled needle with methanol. Draw 1 µL methanol into a glass syringe to fill the needle dead volume and then 1 µL of sample or standard. Spot the sample evenly onto the 5-mm premarked lines until <1 µL of solvent (methanol) remains in the syringe. Allow the plate to dry at ambient temperature (22 °C) after all samples are spotted.
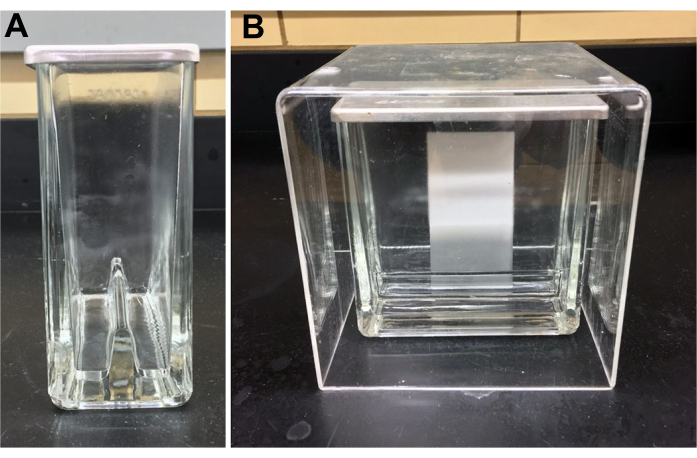
Figure 2: Ganglioside TLC equipment and set up. A twin trough chamber is filled to ≈ 0.5 cm on both sides with running solvent. The plate is placed against one side with the origin end immersed in the running buffer. The chamber is covered with an acrylic box to avoid air currents. Panel A, side view prior to plate insertion. The solvent level is visible a few mm above the chamber bottom; Panel B, front view during development. The solvent front is visible at about 40% of the way up the plate. Please click here to view a larger version of this figure.
- Ganglioside staining
CAUTION: Reagent stains are toxic. Hydrochloric acid is corrosive and toxic. Prepare and spray reagents in a fume hood with protective gloves and safety goggles.
NOTE: The plate can be imaged for qualitative image analysis or stored by removing the clamps and securing the cover plate in place with clear tape. Quantitative analysis can be performed by measuring densitometry of ganglioside standards spotted in adjacent lanes.- Prepare resorcinol spray reagent for the detection of gangliosides based on their sialic acids. Dissolve 6 g of resorcinol in 100 mL water for a 6% resorcinol stock. Dissolve 1 g of CuSO4 in 100 mL of water to make a 1% stock. To 64.7 mL of water add 5 mL of the 6% resorcinol stock, 0.31 mL of the 1% CuSO4 stock then slowly add 30 mL of concentrated HCl and stir gently. May be stored at 4 °C for a month.
- In a chemical fume hood, place the TLC plate with resolved gangliosides, origin end up, in a cut-away cardboard box to protect the walls of the hood from acid spray. Place resorcinol spray reagent in a glass TLC sprayer, attach to a source of pressurized nitrogen, and lightly spray the plate diagonally in the vertical and horizontal directions. Spray the TLC sorbent surface uniformly, but lightly.
- Immediately cover the plate with a clean dry glass cover plate of the same dimensions and secure the cover plate in place with binder clips (Figure 3). Heat the covered plate at 125 °C for 20 min. Gangliosides will appear dark purple against a white background.
NOTE: The plate should not appear wet when spraying is complete. Cover plates can be fashioned by scraping the sorbent from previously used TLC plates using a single-edge razor blade.
CAUTION: Silica powder is toxic to lungs. Use a mask and dispose silica in a sealed container.
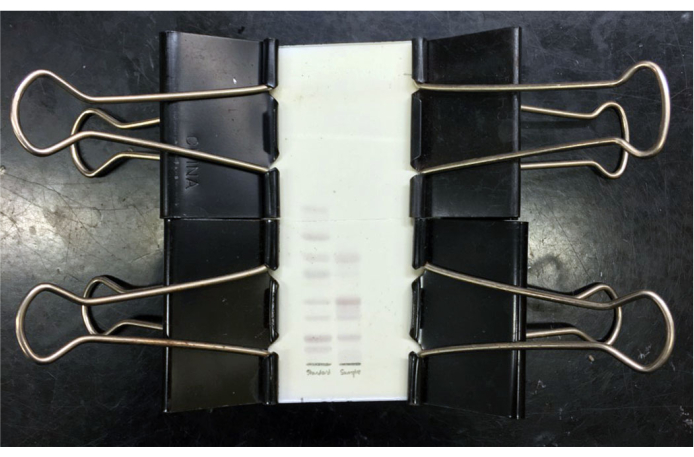
Figure 3: TLC plate of resolved mixed ganglioside. TLC plate of resolved mixed ganglioside standards (left lane) and purified mixed bovine grey matter gangliosides (right lane) after resorcinol staining and heating with glass cover plate clipped in place. Standard gangliosides (top to bottom) are GM3, GM2, GM1, GD3, GD1a, GD1b, GT1b and GQ1b. After cooling, the plate can be imaged and/or the cover plate taped in place for storage. Please click here to view a larger version of this figure.
- General lipid staining for gangliosides and phospholipids.
CAUTION: Sulfuric acid is toxic and corrosive. Addition of concentrated acid to ethanol is exothermic and must be done slowly. Prepare staining reagent in a fume hood with protective gloves and safety goggles.- Prepare p-anisaldehyde stain by slowly adding 15 mL of concentrated sulfuric acid to 500 mL of ethanol. Stir for 30 min to allow the solution to cool before proceeding. Add 15 mL p-anisaldehyde and stir gently. This may be stored at room temperature (22 °C) up to six months.
- In a chemical fume hood, dip the TLC plate with resolved gangliosides, origin end down, into a beaker containing the p-anisaldehyde stain. Submerge to the running front for ≥ 2 s. Remove TLC from stain and allow to drain. Heat the TLC plate on a hot plate at low temperature to develop.
NOTE: Lipids will appear dark against a purple background. Staining solution may be recovered for repeated use.
Results
The methods described in section 1 (small scale) provide gangliosides at sufficient quantity and purity for qualitative and quantitative determination of major brain gangliosides. Recovery from mouse brain is ~ 1 µmol ganglioside per g brain wet weight (1 nmol/µL) when prepared as described. TLC resolution of 1 µL (1 nmol) using section 3 provides ample material for resorcinol detection and resolves all of the major brain gangliosides as shown for wild type and genetically modified mice in
Discussion
The methods for small and large scale ganglioside extraction and isolation reported here are not unique - there are many different solvent extraction and purification approaches that provide excellent results12. The methods reported here for small scale purification from brain, from Fredman and Svennerholm13, were shown to optimize recovery and have proven to be robust and straightforward over many years in our laboratory. Isolation and purification suitable for TLC and MS ...
Disclosures
The authors claim no competing interests.
Acknowledgements
This work was supported by National Institutes of Health (NIH) Common Fund for Glycoscience grant U01CA241953. MJP was supported by the Chemistry-Biology Interface Program at Johns Hopkins (T32GM080189).
Materials
| Name | Company | Catalog Number | Comments |
| Bovine brain, stripped | PelFreez | 57105-1 | |
| Ganglioside standards | Matreya | GM1, 1061; GD1a, 1062; GD1b, 1501; GT1b, 1063 | |
| Glass bottle with PTFE-lined cap | Fisher Scientific | 02-911-739 | |
| Glass centrifuge bottle | Fisher Scientific | 05-586B | |
| Glass culture tubes, 16 x 125 mm | VWR | 60825-430 | for collecting HPLC fractions |
| Glass separatory funnel (2 L) | Pyrex | 6400-2L | |
| Injection syringe - Hamilton 1750 gastight 500 µl | Hamilton | 81265 | |
| p-Anisaldehyde, 98% | Sigma-Aldrich | A88107 | |
| Potter-Elvhjem Homogenizer | Fisher Scientific | 08-414-14A | Choose appropriate volume option |
| Reprosil 100 NH2 10µm 5x4mm guard columns | Analytics-Shop | AAVRS1N-100540-5 | |
| Reprospher 100 NH2, 5 μm, 250 mm x 20 mm HPLC column | Analytics-Shop | custom packed | other sizes available |
| Resorcinol | Sigma-Aldrich | 30752-1 | |
| Rotary evaporator | Buchi | R-300 | |
| Sample loop for Model 7725 Injector (5 ml) | Sigma-Aldrich | 57632 | |
| Sep-Pak tC18 Cartidges Vac 35 cc (10 g) | Waters | WAT043350 | |
| Sep-Pak tC18 Plus Short Cartridge, 400 mg | Waters | WAT036810 | |
| Spotting syringe - Hamilton 701N 10 µl | Hamilton | 80300 | |
| Thick-walled 13-mm diameter test tubes with PFTE lined caps | Fisher Scientific | 14-933A | |
| Threaded 2-ml vials with PFTE lined caps | Fisher Scientific | 14-955-323 | For ganglioside storage |
| TLC plates, HPTLC Silica gel 60 F254 Multiformat | Fisher Scientific | M1056350001 | Fluorescence impregnation (F254) stabilizes the sorbent surface |
| TLC reagent sprayer | Fisher Scientific | 05-723-26A | |
| TLC running chamber for 10 x 10 cm plates | Camag | 22.5155 | |
| Waring 1-Liter Stainless Steal Explosion Resistant Blender | Waring | E8520 |
References
- Schnaar, R. L. The Biology of Gangliosides. Advances in Carbohydrate Chemistry and Biochemistry. 76, 113-148 (2019).
- DeMarco, M. L., Woods, R. J. Atomic-resolution conformational analysis of the GM3 ganglioside in a lipid bilayer and its implications for ganglioside-protein recognition at membrane surfaces. Glycobiology. 19 (4), 344-355 (2009).
- Schnaar, R. L. Gangliosides of the vertebrate nervous system. Journal of Molecular Biology. 428, 3325-3336 (2016).
- Klenk, E. Über die Ganglioside, eine neue Gruppe von zuckerhaltigen Gehirnlipoiden [About gangliosides, a new group of sugar-containing brain lipids]. Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 273, 76-86 (1942).
- Uemura, S., Go, S., Shishido, F., Inokuchi, J. Expression machinery of GM4: the excess amounts of GM3/GM4S synthase (ST3GAL5) are necessary for GM4 synthesis in mammalian cells. Glycoconjugate Journal. 31 (2), 101-108 (2014).
- Nimrichter, L., et al. E-selectin receptors on human leukocytes. Blood. 112 (9), 3744-3752 (2008).
- Saito, M., Kitamura, H., Sugiyama, K. A novel heptasialosyl c-series ganglioside in embryonic chicken brain: its structure and stage-specific expression. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1571 (1), 18-26 (2002).
- Todeschini, A. R., Hakomori, S. I. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1780 (3), 421-433 (2008).
- Sturgill, E. R., et al. Biosynthesis of the major brain gangliosides GD1a and GT1b. Glycobiology. 22, 1289-1301 (2012).
- Cavdarli, S., Delannoy, P., Groux-Degroote, S. O-Acetylated gangliosides as targets for cancer immunotherapy. Cells. 9 (3), (2020).
- Varki, A., et al. Symbol nomenclature for graphical representations of glycans. Glycobiology. 25 (12), 1323-1324 (2015).
- Schnaar, R. L. Isolation of glycosphingolipids. Methods in Enzymology. 230, 348-370 (1994).
- Svennerholm, L., Fredman, P. A procedure for the quantitative isolation of brain gangliosides. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 617, 97-109 (1980).
- Tettamanti, G., Bonali, F., Marchesini, S., Zambotti, V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 296, 160-170 (1973).
- Gazzotti, G., Sonnino, S., Ghidoni, R. Normal-phase high-performance liquid chromatographic separation of non-derivatized ganglioside mixtures. Journal of Chromatography. 348, 371-378 (1985).
- Schnaar, R. L., Needham, L. K. Thin-layer chromatography of glycosphingolipids. Methods in Enzymology. 230, 371-389 (1994).
- Ledeen, R. W., Yu, R. K. Gangliosides: structure, isolation, and analysis. Methods in Enzymology. 83, 139-191 (1982).
- Lopez, P. H., et al. Mice lacking sialyltransferase ST3Gal-II develop late-onset obesity and insulin resistance. Glycobiology. 27 (2), 129-139 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved