A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Partial Heterotopic Hindlimb Transplantation Model in Rats
* These authors contributed equally
In This Article
Summary
This paper presents a partial heterotopic osteomyocutaneous flap transplantation protocol in rats and its potential outcomes in the mid-term follow-up.
Abstract
Vascularized composite allotransplantations (VCA) represent the most advanced reconstruction option for patients without autologous surgical possibilities after a complex tissue defect. Face and hand transplantations have changed disfigured patients' lives, giving them a new aesthetic and functional social organ. Despite promising outcomes, VCA is still underperformed due to life-long immunosuppression comorbidities and infectious complications. The rat is an ideal animal model for in vivo studies investigating immunological pathways and graft rejection mechanisms. Rats are also widely used in novel composite tissue graft preservation techniques, including perfusion and cryopreservation studies. Models used for VCA in rats must be reproducible, reliable, and efficient with low postoperative morbidity and mortality. Heterotopic limb transplantation procedures fulfill these criteria and are easier to perform than orthotopic limb transplants. Mastering rodent microsurgical models requires solid experience in microsurgery and animal care. Herein is reported a reliable and reproducible model of partial heterotopic osteomyocutaneous flap transplantation in rats, the postoperative outcomes, and the means of prevention of potential complications.
Introduction
Over the past two decades, VCA has evolved as a revolutionary treatment for patients who suffer severe disfigurement including face1, upper limb amputations2, penile3, and other complex tissue defects4,5. However, the consequences of life-long immunosuppression still hinder a broader application of these complex reconstructive surgeries. Basic research is crucial to improve anti-rejection strategies. Increasing VCA preservation time is also essential to improve transplantation logistics and increase the donor pool (as VCA donors must fulfill more criteria than solid organ donors, including skin tone, anatomic size, gender). In this context, rat limb transplantations are widely used in studies on the immune rejection of allografts6,7, novel tolerance induction protocols8, and preservation studies9,10,11. Hence, these VCA models are a key element to master for VCA translational research.
Osteomyocutaneous flaps have been described in the literature as reliable models to study VCA in rats8,12,13,14. Although orthotopic whole-limb transplantations allow for long-term evaluation of graft function, it is a time-consuming procedure associated with higher postoperative morbidity and mortality rates14. In contrast, heterotopic limb transplantation models are non-functional, but enable reproducible studies on VCA. Postoperative outcomes can be reliably anticipated before the start of a rat VCA transplantation study. This study reports a partial heterotopic osteomyocutaneous flap transplantation model in the rat that includes frequent possible outcomes and complications that can arise intra-operatively and postoperatively during a follow-up period of three weeks.
Protocol
All animals received humane care in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee (IACUC-protocol 2017N000184) and Animal Care and Use Review Office (ACURO) approved all animal protocols. Inbred male Lewis rats (250-400 g) were used for all experiments.
1. Surgery
- Anesthetize the Lewis rats using isoflurane inhalation. Induce anesthesia with 5% isoflurane in the induction chamber, and maintain anesthesia with 1.5-3% isoflurane inhalation through a breathing cone.
- Apply eye lubricant before surgery in survival procedures. Shave the surgical site, treat with depilatory cream, scrub, and drape with sterile drapes.
- Confirm total anesthesia with a toe pinch test before incision and regularly during the procedure. Monitor heart and respiratory rates throughout the entire procedure. For all surgeries, maintain sterile conditions by using sterile instruments, supplies, drapes, and gloves. See the Table of Materials for the list of instruments used for the procedures.
2. Donor right partial hindlimb procurement
- Make a circumferential incision of the skin above the ankle at the distal third of the leg.
- Skeletonize and cauterize the saphenous artery and the terminal branch of the popliteal artery using bipolar forceps. Cauterize and cut off the gastrocnemius, soleus, tibialis anterior, and biceps femoris muscles until the tibial bone is exposed.
- Make a 2.5 cm incision at the right inguinal crease. Dissect out the inguinal fat pad and retract it distally to expose the femoral vessels. Use a fishhook retractor to grasp the inguinal ligament and clamping forceps to hold the inguinal fat pad distally.
NOTE: The inguinal fat pad is included in the harvest of the partial limb. - Dissect the femoral vessels, individualize Murphy branches (deep muscular collateral branches usually located halfway between the inguinal ligament and the epigastric branch), and ligate with 8-0 nylon ties.
- Heparinize the donor rat with 100 IU/kg heparin, injected in the penile dorsal vein using a 27.5 G needle.
- Complete the skin incision around the hip.
- Cauterize the biceps femoris and gluteus superficialis muscles using bipolar forceps. Cauterize and cut the sciatic nerve at mid femur length. Expose the femur proximally at the level of the posterior femoral crest.
NOTE: Adductor and quadriceps muscles are left out of the procurement. The innominate pedicle is preserved. - Ligate femoral vessels with 8/0 nylon ties at the level of the inguinal ligament. Perform an arteriotomy on the femoral artery just below the ligature and dilate to allow for the insertion of a 24 G angio-catheter.
- Cauterize and cut remaining muscle underneath the pedicle, exposing the anterior side of the femur.
- Cut the tibia and femur using a bone cutter as proximally and distally as possible, respectively (mid-length).
- Flush the partial hindlimb with 2 mL of heparin saline (100 IU/mL) to obtain a clear venous outflow. Store on ice in a sterile gauze until microvascular transfer (Figure 1).
- While the animal is under general anesthesia, perform euthanasia by exsanguination. Confirm death by absence of respiratory movement and heartbeat.

Figure 1: Rat partial hindlimb harvested. A 24 G angiocath is inserted in the femoral artery, ready for heterotopic microvascular transfer. Please click here to view a larger version of this figure.
3. Recipient surgery
- Before the incision, shave the back of the neck, and administer buprenorphine 0.01-0.05 mg/kg subcutaneously. Place the rat in a supine position on a heating pad.
- Make a 2.5 cm incision in the right inguinal crease. Dissect the inguinal fat pad and recline it distally to expose the femoral vessels. Use a hook to retract the inguinal ligament and clamping forceps to hold the inguinal fat pad distally.
- Dissect the femoral vessels, individualize the Murphy branches, and ligate with 8/0 nylon ties.
- Ligate both vessels above the epigastric vessels using 8/0 nylon ties. Place approximator clamps proximally and dilate vessel ends; rinse with heparin saline.
- Make an incision on the left flank above the hip, and create a subcutaneous pocket with a subcutaneous tunnel to the inguinal crease.
NOTE: The inset incision is made above the range of motion of the hip to ensure that the animal maintains a normal hindlimb motion. Additionally, keeping a cutaneous bridge between the graft inset and the microvascular transfer site allows for better fixation of the graft (Figure 2). - Place the proximal part of the partial limb and the inguinal fat pad through the subcutaneous tunnel for microvascular transfer. Perform venous and arterial anastomoses using 10/0 nylon sutures. Remove both approximator clamps, and observe revascularization of the limb. Perform a "milking test" on both vessels to assess the patency of each anastomosis.
NOTE: Eight to nine sutures are usually necessary for venous anastomosis, 6 sutures on average for arterial anastomosis. - Make a longitudinal skin incision on the medial side of the transplanted limb, and insert the graft. Remove excess skin of the graft, and close the wound with separate sutures and a running suture using absorbable 4/0 sutures.
- Suture together the inguinal fat pads of the transplanted limb and the recipient using two separate absorbable sutures, and close the inguinal crease at the very end after a last checkup of the microvascular anastomoses.
NOTE: Inguinal fat pads are sutured tightly to add a protective layer of fat above the anastomoses and ensure a secured position of the graft and its pedicle. A meticulous closure is better for wound healing; it also prevents residual bleeding from the wound and decreases the risk of self-mutilation. - Compensate fluid loss subcutaneously with 1-3 mL of saline according to the amount of perioperative bleeding.
- Place an Elizabethan collar around the neck of the animal, and apply 2 loose sutures to the skin to maintain it in the correct position.
- Stop isoflurane inhalation, and monitor the animal continuously on a warming pad until fully conscious and ambulatory.
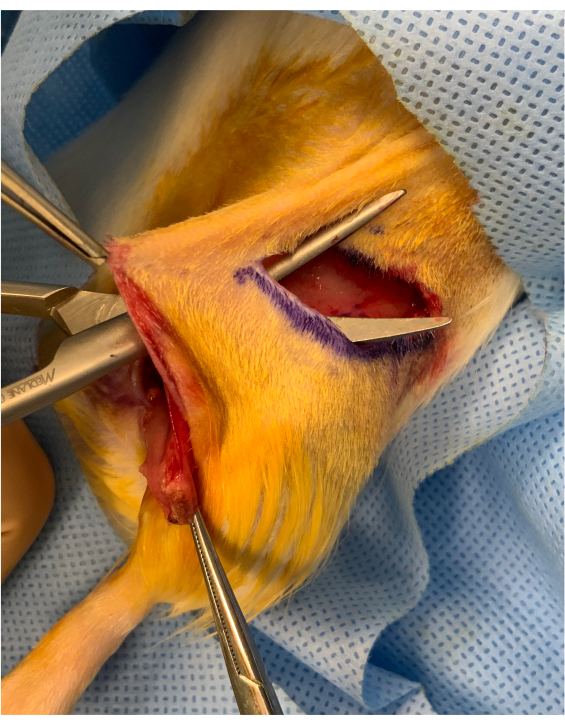
Figure 2: Perioperative image before inset of the osteomyocutaneous limb. A cutaneous bridge of approximately 1 cm is preserved between the inguinal crease incision and the inset of the graft above the hip. The graft is placed under the bridge, maintaining it steady for microvascular transfer. Please click here to view a larger version of this figure.
4. Postoperative care
- Monitor the animal twice daily for 72 hours, then once daily until postoperative day (POD) 7, and then twice per week.
NOTE: Monitoring must be adjusted to the animal and graft condition (pale eyes might require supplementary fluids, porphyrin staining as an indicator of animal pain, abnormal graft color/temperature), and further care should be discussed with the veterinarian. Single housing is required for the recipient rats during the entire study period to avoid any damage to the graft. - Perform analgesia with subcutaneous injection of buprenorphine and/or non-steroid anti-inflammatory drug according to IACUC guidelines.
- Evaluate the graft, and perform physical examination daily with pictures using the same device.
NOTE: Using hair removal cream on the graft's skin is helpful to better assess the skin color of the transplant.
Results
In this single-operator study, 30 syngeneic heterotopic partial limb transplants were performed. Success was defined at postoperative day 21 as the absence of VCA failure or complications requiring euthanasia. The normal evolution of the graft is represented in Figure 3. The mean duration for partial limb procurement and graft inset in the recipient were 35 and 105 min, respectively; the mean ischemia time was 105 min. During follow-up, two types of complications occurred (Table 1
Discussion
Orthotopic limb transplantation models in rodents have been described in the literature16,17,18; however, they require a nerve repair, muscle reattachment, and a perfect osteosynthesis of the femur, which can be a very difficult step. These models are also associated with a higher morbidity and mortality rate in rodents14, especially in the short-term follow-up as the recovery of a normal function of a tr...
Disclosures
The authors have no disclosures.
Acknowledgements
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Congressionally Directed Medical Research Program under Award No. W81XWH-17-1-0680. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Materials
| Name | Company | Catalog Number | Comments |
| 24 GA angiocatheter | BD Insyte Autoguard | 381412 | |
| 4-0 suture Black monofilament non absorbable suture | Ethicon | 1667 | Used to suture the E-collar to the back of the neck |
| 4-0 suture Coated Vicryl Plus Antibacterial | Ethicon | VCP496 | |
| Adson Tissue Forceps, 11 cm, 1 x 2 Teeth with Tying Platform | ASSI | ASSI.ATK26426 | |
| Bipolar cords | ASSI | 228000C | |
| Black Polyamide Monofilament USP 10-0, 4 mm 3/8c | AROSurgical | T04A10N07-13 | Used to perform the microvascular anastomoses |
| Buprenorphine HCl | Pharmaceutical, Inc | 42023-179-01 | |
| Dilating Forceps | Fine science tools (FST) | 18131-12 | |
| Dissecting Scissors 15 cm, Round Handle 8 mm diameter, Straight Slender Tapered Blade 7 mm, Lipshultz Pattern | ASSI | ASSI.SAS15RVL | |
| Double Micro Clamps 5.5 x 1.5 mm | Fine science tools (FST) | 18040-22 | |
| Elizabethan collar | Braintree Scientific | EC-R1 | |
| Forceps 13.5 cm long, Flat Handle, 9 mm wide Straight Tips 0.1 mm diameter (x2) | ASSI | ASSI.JFL31 | |
| Halsey Micro Needle Holder | Fine science tools (FST) | 12500-12 | |
| Heparin Lock Flush Solution, USP, 100 units/mL | BD PosiFlush | 306424 | |
| Isoflurane | Patterson Veterinary | 14043-704-06 | |
| Jewelers Bipolar Forceps Non Stick 11 cm, straight pointed tip, 0.25 mm tip diameter | ASSI | ASSI.BPNS11223 | |
| Lone Star Elastic Stays | CooperSurgical | 3314-8G | Used to retract the inguinal ligament for femoral vessels dissection |
| Lone Star Self-Retaining Retractors | CooperSurgical | 3301G | |
| Micro-Mosquito Hemostats | Fine science tools (FST) | 13010-12 | Used to retract the inguinal fat pad distally |
| Needle Holder, 15 cm Round Handle, 8 mm diameter, Superfine Curved Jaw 0.2 mm tip diameter, without lock | ASSI | ASSI.B1582 | |
| Nylon Suture Black Monolfilament 8-0, 6.5 mm 3/8c | Ethilon | 2808G | Used to ligate collateral branches on the femoral vessels |
| Offset Bone Nippers | Fine science tools (FST) | 16101-10 | |
| S&T Vascular Clamps 5.5 x 1.5 mm | Fine science tools (FST) | 00398-02 | |
| Walton scissors | Fine science tools (FST) | 14077-09 |
References
- Lanteiri, L., et al. Feasibility, reproducibility, risks and benefits of face transplantation: a prospective study of outcomes. American Journal of Transplantation. 11 (2), 367-378 (2011).
- Park, S. H., Eun, S. C., Kwon, S. T. Hand transplantation: current status and immunologic obstacles. Experimental and Clinical Transplantation. 17 (1), 97-104 (2019).
- Cetrulo, C. L., et al. Penis transplantation: first US experience. Annals of Surgery. 267 (5), 983-988 (2018).
- Grajek, M., et al. First complex allotransplantation of neck organs: larynx, trachea, pharynx, esophagus, thyroid, parathyroid glands, and anterior cervical wall: a case report. Annals of Surgery. 266 (2), 19-24 (2017).
- Pribaz, J. J., Caterson, E. J. Evolution and limitations of conventional autologous reconstruction of the head and neck. Journal of Craniofacial Surgery. 24 (1), 99-107 (2013).
- Lipson, R. A., et al. Vascularized limb transplantation in the rat. I. Results with syngeneic grafts. Transplantation. 35 (4), 293-299 (1983).
- Lipson, R. A., et al. Vascularized limb transplantation in the rat. II. Results with allogeneic grafts. Transplantation. 35 (4), 300-304 (1983).
- Adamson, L. A., et al. A modified model of hindlimb osteomyocutaneous flap for the study of tolerance to composite tissue allografts. Microsurgery. 27 (7), 630-636 (2007).
- Arav, A., Friedman, O., Natan, Y., Gur, E., Shani, N. Rat hindlimb cryopreservation and transplantation: a step toward "organ banking". American Journal of Transplantation. 17 (11), 2820-2828 (2017).
- Gok, E., et al. Development of an ex-situ limb perfusion system for a rodent model. ASAIO Journal. 65 (2), 167-172 (2019).
- Gok, E., Rojas-Pena, A., Bartlett, R. H., Ozer, K. Rodent skeletal muscle metabolomic changes associated with static cold storage. Transplantation Proceedings. 51 (3), 979-986 (2019).
- Brandacher, G., Grahammer, J., Sucher, R., Lee, W. P. Animal models for basic and translational research in reconstructive transplantation. Birth Defects Research. Part C, Embryo Today. 96 (1), 39-50 (2012).
- Fleissig, Y., et al. Modified heterotopic hindlimb osteomyocutaneous flap model in the rat for translational vascularized composite allotransplantation research. Journal of Visualized Experiments: JoVE. (146), e59458 (2019).
- Ulusal, A. E., Ulusal, B. G., Hung, L. M., Wei, F. C. Heterotopic hindlimb allotransplantation in rats: an alternative model for immunological research in composite-tissue allotransplantation. Microsurgery. 25 (5), 410-414 (2005).
- Jang, Y., Park, Y. E., Yun, C. W., Kim, D. H., Chung, H. The vest-collar as a rodent collar to prevent licking and scratching during experiments. Lab Anim. 50 (4), 296-304 (2016).
- Kern, B., et al. A novel rodent orthotopic forelimb transplantation model that allows for reliable assessment of functional recovery resulting from nerve regeneration. American Journal of Transplantation. 17 (3), 622-634 (2017).
- Perez-Abadia, G., et al. Low-dose immunosuppression in a rat hind-limb transplantation model. Transplant International. 16 (12), 835-842 (2003).
- Sucher, R., et al. Orthotopic hind-limb transplantation in rats. Journal of Visualized Experiments. (41), e2022 (2010).
- Fleissig, Y. Y., Beare, J. E., LeBlanc, A. J., Kaufman, C. L. Evolution of the rat hind limb transplant as an experimental model of vascularized composite allotransplantation: Approaches and advantages. SAGE Open Medicine. 8, 2050312120968721 (2020).
- Lindboe, C. F., Presthus, J. Effects of denervation, immobilization and cachexia on fibre size in the anterior tibial muscle of the rat. Acta Neuropathologica. 66 (1), 42-51 (1985).
- Nazzal, J. A., Johnson, T. S., Gordon, C. R., Randolph, M. A., Lee, W. P. Heterotopic limb allotransplantation model to study skin rejection in the rat. Microsurgery. 24 (6), 448-453 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved