A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Reconstitution of Msp1 Extraction Activity with Fully Purified Components
In This Article
Summary
Here, we present a detailed protocol for reconstitution of Msp1 extraction activity with fully purified components in defined proteoliposomes.
Abstract
As the center for oxidative phosphorylation and apoptotic regulation, mitochondria play a vital role in human health. Proper mitochondrial function depends on a robust quality control system to maintain protein homeostasis (proteostasis). Declines in mitochondrial proteostasis have been linked to cancer, aging, neurodegeneration, and many other diseases. Msp1 is a AAA+ ATPase anchored in the outer mitochondrial membrane that maintains proteostasis by removing mislocalized tail-anchored proteins. Using purified components reconstituted into proteoliposomes, we have shown that Msp1 is necessary and sufficient to extract a model tail-anchored protein from a lipid bilayer. Our simplified reconstituted system overcomes several of the technical barriers that have hindered detailed study of membrane protein extraction. Here, we provide detailed methods for the generation of liposomes, membrane protein reconstitution, and the Msp1 extraction assay.
Introduction
Proper cellular function depends upon a process called proteostasis, which ensures that functional proteins are at the correct concentration and cellular location1. Failures in proteostasis lead to compromised organelle function and are associated with many neurodegenerative diseases2,3,4. Membrane proteins present unique challenges to the proteostasis network as they must be targeted to the correct membrane while avoiding aggregation from the hydrophobic transmembrane domains (TMDs)5. Consequently, specialized machinery has evolved to shield the hydrophobic TMD from the cytosol and facilitate targeting and insertion into the proper cellular membrane6,7,8,9,10,11,12,13,14,15.
Mitochondria are the metabolic hub of the cell and are involved in numerous essential cellular processes such as: oxidative phosphorylation, iron-sulfur cluster generation, and apoptotic regulation16,17. These endosymbiotic organelles contain two membranes, referred to as the inner mitochondrial membrane (IMM) and the outer mitochondrial membrane (OMM). Over 99% of the 1,500 human mitochondrial proteins are encoded in the nuclear genome and need to be translocated across one or two different membranes18,19. Proper mitochondrial function thus depends on a robust proteostasis network to correct any errors in protein targeting or translocation.
Our lab focuses on a subset of mitochondrial membrane proteins called tail-anchored (TA) proteins, which have a single transmembrane domain at the very C-terminus20,21,22,23,24. TA proteins are involved in a number of essential processes, such as apoptosis, vesicle transport, and protein translocation25. The unique topology of TA proteins requires post-translational insertion, which occurs in the endoplasmic reticulum (ER) by the Guided Entry of Tail-anchored (GET) or Endoplasmic reticulum Membrane protein Complex (EMC) pathways or into the OMM by a poorly characterized pathway20,26,27,28. The biophysical properties of the TMD are necessary and sufficient to guide TA proteins to the correct membrane29. The recognition of biophysical characteristics rather than a defined sequence motif limits the fidelity of the targeting pathways5. Thus, mislocalization of TA proteins is a common stress for the proteostasis networks. Cellular stress, such as inhibition of the GET pathway, causes an increase in protein mislocalization to the OMM and mitochondrial dysfunction unless these proteins are promptly removed30,31.
A common theme in membrane proteostasis is the use of AAA+ (ATPase Associated with cellular Activities) proteins to remove old, damaged, or mislocalized proteins from the lipid bilayer1,32,33,34,35,36,37,38. AAA+ proteins are molecular motors that form hexameric rings and undergo ATP dependent movements to remodel a substrate, often by translocation through a narrow axial pore39,40. Although great effort has been devoted to studying the extraction of membrane proteins by AAA+ ATPases, the reconstitutions are complex or involve a mixture of lipids and detergent41,42, which limits the experimental power to examine the mechanism of substrate extraction from the lipid bilayer.
Msp1 is a highly conserved AAA+ ATPase anchored in the OMM and peroxisomes that plays a critical role in membrane proteostasis by removing mislocalized TA proteins43,44,45,46,47. Msp1 was also recently shown to alleviate mitochondrial protein import stress by removing membrane proteins that stall during translocation across the OMM48. Loss of Msp1 or the human homolog ATAD1 results in mitochondrial fragmentation, failures in oxidative phosphorylation, seizures, increased injury following stroke, and early death31,49,50,51,52,53,54,55,56.
We have shown that it is possible to co-reconstitute TA proteins with Msp1 and detect the extraction from the lipid bilayer57. This simplified system uses fully purified proteins reconstituted into defined liposomes which mimic the OMM (Figure 1)58,59. This level of experimental control can address detailed mechanistic questions of substrate extraction that are experimentally intractable with more complex reconstitutions involving other AAA+ proteins. Here, we provide experimental protocols detailing our methods for liposome preparation, membrane protein reconstitution, and the extraction assay. It is our hope that these experimental details will facilitate further study of the essential but poorly understood process of membrane proteostasis.
Protocol
1. Liposome Preparation
- Combine chloroform stocks of lipids in appropriate ratios to mimic the outer mitochondrial membrane.
- Prepare 25 mg of lipid mixture. We use a previously established mixture of lipids that mimic mitochondrial membranes, consisting of a 48:28:10:10:4 molar ratio of chicken egg phosphatidyl choline (PC), chicken egg phosphatidyl ethanolamine (PE), bovine liver phosphatidyl inositol (PI), synthetic 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS), and synthetic 1',3'-bis[1,2-dioleoyl-sn-glycero-3-phospho]-glycerol (TOCL)58,59. Sample calculations are shown in Table 1.
- Bring all lipid stocks to room temperature before opening as this will limit condensation. As most labs do not have a precise way to measure the concentration of the lipids, any water absorbed by the chloroform stock will change the concentration of the lipid stock and thus the ratio of lipids used in the assay.
- As lipid stocks come in glass ampules, transfer the required amount of lipid to a glass vial using a 1 mL syringe. Add 2 mg of dithiothreitol (DTT) to the vial to prevent lipid oxidation. Work quickly as evaporation of chloroform will change the concentration of the lipid.
- Transfer any remaining lipid to a separate glass vial and fit with a PFTE septa. Add 2 mg of DTT to the vial, wrap with parafilm and store at -20 °C to prevent lipid oxidation. Try to use the lipids within 3 months of transfer to the vials. To prevent potential contamination of the chloroform stocks by marker runoff, transfer the stickers from the original ampules to the glass vials rather than label with marker.
- Evaporate chloroform under a very gentle stream of nitrogen while spinning the glass vial continuously by hand in a fume hood, essentially acting as a manual rotovap. Spin the vial at a consistent speed (20-40 rpm) by hand to keep the lipids moving. The goal is to evaporate all the chloroform and get an even coating of lipids over the entire glass vial.
CAUTION: Chloroform is neurotoxic, and this step should be performed in the fume hood.- Attach a fresh Pasteur pipette to the nitrogen tube. Do not let any of the lipids splash out of the vial or onto the Pasteur pipette. Aim the tip at the bottom of the vial so the air bounces off the bottom and pushes the lipids up towards the center of vial. Get an even coating over the entire vial while avoiding any accumulation in the corners or by the cap. This whole process takes about 5 minutes.
- As the mixture thickens into a "bead" of lipids, guide it into the center of the vial by changing the angle of the vial. Once the bead starts to become smaller, turn up the nitrogen stream slightly to disperse the bead, ensuring that none of the lipids blow out of the vial.
- Remove any remaining chloroform under vacuum.
- Put the glass vial on a house vacuum or diaphragm vacuum pump for 1 hour to remove the majority of residual chloroform. These vacuums are generally not strong enough to remove all of the chloroform, but they can tolerate small amounts of solvent better than rotary-vain vacuums.
- Put vial on a strong vacuum (<1 mTorr) for 12-16 h to remove all residual chloroform. Be sure to avoid bumping of the vial during this process.
- Resuspend the lipids in 1.25 mL of liposome buffer (50 mM HEPES KOH pH 7.5, 15% glycerol, 1 mM DTT). As we started with 25 mg of lipids, this results in a concentration of 20 mg/mL. Fully resuspend the lipids with no visible chunks. If the lipids pooled in the corner of the vial, this can be a lengthy process.
- Vortex the vial vigorously until the sample is milky smooth. We find that if the chloroform evaporation was done properly the night before, this process takes about 5-10 minutes.
- To ensure complete resuspension of the lipids, rotate on a wheel at room temperature for 3 hours at ~80 rpm. Remove vial from the wheel once every hour for 1 minute of vortexing to ensure even mixing.
- Transfer lipids carefully to a clean 1.5 mL microcentrifuge tube. Perform 5 freeze-thaw cycles using liquid nitrogen to freeze and a 30 °C heat block to thaw. This step helps to convert multilamellar vesicles to unilamellar vesicles.
- Extrude the lipids.
- During the freeze thaw cycles, prepare the mini-extruder. Assemble the mini-extruder with 10 mm filter supports and a polycarbonate membrane of the desired pore size (we use 200 nm). The size of the filter will affect the size of the liposome, which will affect the concentration of proteins required for the reconstitution (Step 2.3).
- Place the mini-extruder onto a hot plate and bring the extruder temperature up to 60 °C.
- Draw up the lipids into a 1 mL gas-tight glass syringe and carefully place into one end of the mini-extruder. Place the empty gas-tight syringe into the other side of the mini-extruder. Allow the lipids to equilibrate to the extruder stand temperature for 5-10 minutes.
- Transfer the lipids to the alternate syringe by gently pushing the plunger of the filled syringe. Push the solution from the alternate syringe into the original syringe. Repeat this back-and-forth process 15 times, so that at the 15th pass the lipids end in the alternate syringe. Monitor the volume in each pass to make sure that there are no leaks.
- Prepare single use aliquots of the lipids, flash freeze in liquid nitrogen and store at -80 °C. The liposomes are stable at -80 °C for several months. The reconstitutions require 10 μL of liposomes at a time (Step 2.3.4), so it is convenient to prepare 10 μL or 20 μL aliquots.
2 Reconstitution of Msp1 and Model TA protein
- Prepare the reconstitution buffer: 50 mM HEPES pH 7.5, 200 mM potassium acetate, 7 mM magnesium acetate, 2 mM DTT, 10% sucrose, 0.01% sodium azide, 0.2-0.8% Deoxy Big Chaps (DBC).
- Optimize reconstitution conditions for the new batch of liposomes. The concentration of DBC and biobeads required for optimal reconstitution varies depending on the batch of liposomes used. To limit prep to prep variability, use the same lot of DBC for all experiments. When changing lots of DBC, repeat the optimization process.
- Set up a series of reconstitutions with different concentrations of DBC (0.2% - 0.8%) and biobeads (25 mg - 100 mg) each time a new batch of liposomes is prepared. It is important to not drop the DBC below the critical micelle concentration (CMC) of ~0.12%. Once conditions are optimized, we recommend collecting all data using the same liposome prep and reconstitution conditions.
- Assay the effectiveness of the various reconstitution conditions by using the extraction assay described in Step 3.
- Prepare biobeads at a final concentration of 250 mg/mL.
- Weigh out 2.7 g of dried biobeads and resuspend in a 50 mL centrifuge tube of 100% methanol (about 45 mL) to wet the beads. Initially wet the biobeads in methanol to prevent air from being trapped in the pores of the beads. Once in methanol, keep the biobeads wet as any air trapped by the biobeads will alter their ability to absorb detergent.
- Remove methanol by washing the beads 8x with about 45 mL of ultrapure water (18.2 mΩ), hereafter referred to as ddH2O. Pellet beads by spinning at 3,200 x g for 1 minute. Decant the liquid and resuspend in ddH2O.
- After washing, resuspend in 10 ml of ddH2O with 0.02% sodium azide and store at 4 °C. Biobeads can be stored at 4 °C for several months. This stock is 250 mg/mL as it is assumed ~0.2 g is lost during the wash steps.
- Calculate the size of the liposome and the desired number of molecules of TA protein and Msp1 per liposome. This will determine the concentration of Msp1 and TA protein required for the reconstitution.
- First, calculate the number of lipid molecules per unilamellar liposome (Ntotal) using the equation
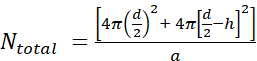 where d is the diameter of the liposome, h is the thickness of the bilayer, and a is the lipid headgroup area.
where d is the diameter of the liposome, h is the thickness of the bilayer, and a is the lipid headgroup area.
- Measure the liposome diameter by DLS. In our example, a value of 70 nm for the liposome diameter (d) was obtained.
- Use a value of 5 nm for h and 0.71 nm2 for a, which is the headgroup size for phosphatidylcholine. In this particular situation, Ntotal is 37,610.
- Next, calculate the molar concentration of lipid MLipid using the average molecular weight of the lipids in the mixture. In this example, the concentration of lipids is 20 mg/mL (Step 1.4) and the average molecular weight of lipids is 810 g/mol (Table 1). This results in a value of 0.0247 M for MLipid.
- Next, calculate the molar concentration of liposomes, MLiposome, using the equation
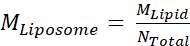 where MLipid is the molar concentration of lipid from Step 2.3.2, and Ntotal is the total number of lipids per liposome calculated in Step 2.3.1. In this example, the 20 mg/mL stock concentration of liposomes is approximately 660 nM.
where MLipid is the molar concentration of lipid from Step 2.3.2, and Ntotal is the total number of lipids per liposome calculated in Step 2.3.1. In this example, the 20 mg/mL stock concentration of liposomes is approximately 660 nM. - Calculate the amount of Msp1 and TA protein required for a 100 μL reconstitution reaction.
NOTE: The final concentration of lipids in the reconstitution is 2 mg/mL, which is a 10x dilution of the liposome stock. This gives a final liposome concentration of 66 nM. The final concentration of Msp1 is 792 nM, which gives an average of 12 total copies (2 functional hexamers) per liposome. The final concentration of TA protein is 660 nM, which gives an average of 10 copies per liposome.
- First, calculate the number of lipid molecules per unilamellar liposome (Ntotal) using the equation
- In a PCR tube, mix together purified Msp1, TA protein, and liposomes in reconstitution buffer. The order of addition is buffer, proteins, and liposomes last. The total volume is 100 μL. Allow the mixture to sit on ice for 10 minutes. Purify the Msp1 and TA protein as previously described57.
- Use the well-characterized model TA protein His-Flag-Sumo-Sec22 as a positive control substrate when first establishing the assay. This construct has a His-tag for easy purification, 3x-Flag tag for detection by western blot, a Sumo domain for increased solubility, and the TMD of the ER-TA protein Sec22 for reconstitution and recognition by Msp1.
- Ensure that stock solutions of both Msp1 and the TA protein are approximately 100 μM to minimize the effect of N-Dodecyl β-D-maltoside (DDM) from the protein purification on the reconstitution. Purified Msp1 is in 20 mM HEPES pH 7.5, 100 mM sodium chloride, 0.1 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP), 0.05% DDM whereas the purified TA protein is in 50 mM Tris pH 7.4, 150 mM sodium chloride, 10 mM magnesium chloride, 5 mM β-mercaptoethanol, 10% glycerol, 0.1% DDM. Stock solutions of approximately 100 μM TA protein and Msp1 monomer ensures that these components will make up < 5% of the final reconstitution volume, resulting in a dilution of DDM below the CMC.
- Add the desired amount of biobeads to the sample to remove detergent.
- Cut the tip of a p200 pipette tip to about 1/8th inch in diameter so that beads can fit through the tip. Vortex the biobeads tube thoroughly to obtain a uniform mixture and quickly remove the lid and pipette up the volume before the biobeads settle. Transfer the biobeads to an empty PCR tube.
- When the reconstitution has finished its 10-minute incubation on ice, use an uncut pipette tip to remove all of the liquid from the biobeads. Then transfer the 100 μL reconstitution into the tube with the biobeads. This must be done quickly so that the biobeads do not trap air, which will cause the beads to float.
- Allow the reconstitution to rotate on a wheel at ~80 rpm for 16 hours at 4 °C.
- Remove reconstituted material from biobeads. Do a quick spin in a picofuge to pellet the biobeads, and then use an uncut pipet tip to transfer the reconstituted material to a clean PCR tube. Repeat this process 1-2 times until there are no biobeads left in the sample. Keep reconstitution on ice.
- Pre-clear the reconstituted material to remove any proteins which failed to reconstitute into the liposomes.
- Prepare Extraction Buffer: 50 mM HEPES pH 7.5, 200 mM potassium acetate, 7 mM magnesium acetate, 2 mM DTT, 100 nM calcium chloride.
- Equilibrate the glutathione spin columns with Extraction Buffer according to the manufacturer’s directions. This typically involves 3-rounds of washing with 400 μL of buffer and then centrifuging at 700 x g for 2 minutes at room temperature to remove the buffer.
- Add 5 μM of each chaperone (GST-SGTA and GST-Calmodulin) to the reconstituted material. These chaperones will bind to the TMD of any proteins which failed to reconstitute into the liposomes. Purification of chaperones was described previously6,57.
NOTE: These chaperones are commercially available, but we prefer to purify in house to control for cost and quality. Both proteins are in 20 mM Tris pH 7.5, 100 mM sodium chloride, and 0.1 mM TCEP, and have a stock concentration around 160 μM. SGTA recognizes substrates with a highly hydrophobic TMD whereas Calmodulin binds TMDs with moderate hydrophobicity6,29. Together, this chaperone cocktail can recognize a broad range of substrates. - Add 100 μL of extraction buffer to the reconstituted material, bringing the volume up to 200 μL. Add this to the equilibrated glutathione spin columns. Note that the glutathione spin columns provide the highest sample recovery when the pre-clearing volume is 200 – 400 μL.
- Plug the spin columns and rotate at ~80 rpm at 4 °C for 30 minutes to allow chaperones to bind to resin.
- Spin the columns at 700 x g for 2 minutes at room temperature. The flow through is pre-cleared material that is depleted of aggregated proteins. Keep material on ice and proceed directly with extraction assay.
3. Extraction Assay
- Prepare tubes for SDS PAGE analysis. Each reaction will have 4 tubes: INPUT (I), FLOW THROUGH (FT), WASH (W), and ELUTE (E).
- Add 45 μL of ddH2O sample to the INPUT tube, 40 μL of ddH2O to the FLOW THROUGH tube, and 0 μL to the WASH and ELUTE tubes.
NOTE: The best signal to noise ratio in this assay is obtained when the WASH and ELUTE samples are 5x concentrated relative to the INPUT and FLOW THROUGH samples. Due to the dilutions during the assay, this requires taking 5 μL of sample for the INPUT sample, 10 μL of sample for the FLOW THROUGH sample, and 50 μL of sample for the WASH and ELUTE samples. - Add 16.6 μL of 4x SDS PAGE Loading Buffer to each tube. The total volume of each sample is 50 μL before SDS PAGE Loading Buffer. The final volume is 66.6 μL (50 μL sample + 16.6 μL of 4x SDS PAGE Loading Buffer).
- Add 45 μL of ddH2O sample to the INPUT tube, 40 μL of ddH2O to the FLOW THROUGH tube, and 0 μL to the WASH and ELUTE tubes.
- Assemble the extraction assay.
- Prepare the extraction reaction containing 60 μL of pre-cleared proteoliposomes, 5 μM of GST-SGTA, 5 μM of GST-Calmodulin, and 2 mM ATP. Combine all reagents except ATP, which is used to initiate the reaction. Bring to a final volume of 200 μL with Extraction Buffer.
NOTE: As 60 μL of sample are used for each extraction assay, one reconstitution can be used for three different extraction assays. Perform positive and negative controls (+ATP and -ATP) on material from the same reconstitution. - Pre-warm extraction assay in 30 °C heat block for 2 minutes.
- Prepare the extraction reaction containing 60 μL of pre-cleared proteoliposomes, 5 μM of GST-SGTA, 5 μM of GST-Calmodulin, and 2 mM ATP. Combine all reagents except ATP, which is used to initiate the reaction. Bring to a final volume of 200 μL with Extraction Buffer.
- Initiate the extraction assay by adding ATP to final concentration of 2 mM and start timer.
- Give a 5 second spin in a picofuge to mix ATP into the reaction. Incubate reaction at 30 °C for 30 minutes.
- During the incubation, take 5 μL of the reaction and add to the INPUT tube. The timing of this is flexible.
- During this incubation period, equilibrate one glutathione spin column for each sample in the extraction assay.
- Perform pull down on chaperones to isolate extracted material.
- Once the 30-minute incubation is finished, add 200 μl of extraction buffer to the tube to bring total volume to 400 μL. Add to equilibrated glutathione resin and allow to bind on wheel at 4° C for 30 minutes.
- Spin the columns at 700 x g for 2 minutes at room temperature to collect the flow through. Take 10 μL for the FLOW THROUGH tube. This sample contains substrates which are still integrated in the lipid bilayer.
- Wash resin twice with 400 μL of extraction buffer, discarding the flow through. On the third wash, keep the flow through and take 50 μL for the WASH tube.
- Prepare 5 mL of Elution Buffer by adding reduced glutathione to a final concentration of 5 mM in Extraction Buffer. Prepare this buffer fresh each time.
- Add 200 μL of Elution Buffer to the spin column. Incubate at room temperature for 5 minutes. Spin at 700 x g for 2 minutes at room temperature to elute. Keep the flow through. Repeat the process a second time so that the total elution volume is 400 μL.
- Take 50 μL of sample from the Elution sample and add it to the ELUTE tube.
- Analyze extraction activity using SDS-PAGE and western blot.
NOTE: As a western blot is a fairly standard procedure, a basic protocol is provided that highlights a few of the details unique to this assay.- Load samples into a stain free polyacrylamide gel (4% stacking, 15% separating) and run at 200 V for 50 minutes in Tris-glycine buffer. If space permits, use both an unstained and stained ladder to permit visualization on stain-free gel imager and transfer to PVDF membrane respectively. The stain free gel allows quantitative visualization of tryptophan containing proteins upon activation with ultraviolet light, while still allowing the gel to be used for a western blot.
- Image the stain free gel to confirm that there is equal loading across all samples. This is an essential control that ensures any changes in signal detected by western blot are not a result of variable protein loading. There should only be visible bands for the chaperones (GST-calmodulin and GST-SGTA) in the INPUT and ELUTION samples. Recall that the ELUTE sample will be more concentrated than the INPUT sample.
- Assemble a western blot cassette using a 45 μm PVDF membrane. Transfer at a constant current of 300 mA for 60 minutes.
- After blocking the membrane, bind to the primary antibody for 16 h at 4 °C with gentle shaking ~15 rpm. Blot for substrate with rabbit Anti-FLAG at a 1:1,000 dilution.
NOTE: The primary and secondary antibodies used will be substrate specific and the concentration for use may need to be optimized. - Wash membrane and incubate with secondary antibody, goat anti-rabbit at a 1:10,000 dilution, with gentle shaking for 1 hour at room temperature.
- Wash membrane and image for analysis using western blotting detection agent.
Results
To properly interpret the results, the stain free gel and the western blot must be viewed together. The stain free gel ensures equal loading across all samples. When viewing the stain free gel, the chaperones (GST-calmodulin and GST-SGTA) will be visible in the INPUT (I) and ELUTE (E) lanes. Double check that the intensity of these bands is uniform across all of the INPUT samples. Likewise, ensure that the intensity is uniform across the ELUTE samples. The ELUTE is 5x more concentrated than the INPUT and this difference ...
Discussion
Proper mitochondrial function depends upon a robust protein quality control system. Due to inherent limits in the fidelity of the TA protein targeting pathways, mislocalized TA proteins are a constant source of stress for mitochondria. A key component of the mitochondrial proteostasis network is Msp1, which is a membrane anchored AAA+ ATPase that removes mislocalized TA proteins from the OMM. Here, we have described how to prepare proteoliposomes, co-reconstitute Msp1 and a model TA protein, and perform an extraction ass...
Disclosures
None
Acknowledgements
MLW developed part of this protocol during his postdoctoral studies with Dr. Robert Keenan at the University of Chicago.
This work is funded by NIH grant 1R35GM137904-01 to MLW.
Materials
| Name | Company | Catalog Number | Comments |
| Biobeads | Bio-Rad | 1523920 | |
| Bovine liver phosphatidyl inositol | Avanti | 840042C | PI |
| Chicken egg phosphatidyl choline | Avanti | 840051C | PC |
| Chicken egg phosphatidyl ethanolamine | Avanti | 840021C | PE |
| ECL Select western blotting detection reagent | GE | RPN2235 | |
| Filter supports | Avanti | 610014 | |
| Glass vial | VWR | 60910L-1 | |
| Glutathione spin column | Thermo Fisher | PI16103 | |
| Goat anti-rabbit | Thermo Fisher | NC1050917 | |
| Mini-Extruder | Avanti | 610020 | |
| Polycarbonate membrane | Avanti | 610006 | 200 nM |
| PVDF membrane | Thermo Fisher | 88518 | 45 µM |
| Rabbit anti-FLAG | Sigma-Aldrich | F7245 | |
| Synthetic 1,2-dioleoyl-sn-glycero-3-phospho-L-serine | Avanti | 840035C | DOPS |
| Synthetic 1',3'-bis[1,2-dioleoyl-sn-glycero-3-phospho]-glycerol | Avanti | 710335C | TOCL |
| Syringe, 1 mL | Norm-Ject | 53548-001 | |
| Syringe, 1 mL, gas-tight | Avanti | 610017 |
References
- Song, J., Herrmann, J. M., Becker, T. Quality control of the mitochondrial proteome. Nature Reviews Molecular Cell Biology. 22, 54-70 (2021).
- Phillips, B. P., Miller, E. A. Membrane protein folding and quality control. Current Opinion in Structural Biology. 69, 50-54 (2021).
- Jiang, H. Quality control pathways of tail-anchored proteins. Biochimica et Biophysica Acta - Molecular Cell Research. 1868, 118922 (2020).
- McKenna, M. J., et al. The endoplasmic reticulum P5A-ATPase is a transmembrane helix dislocase. Science. 369, (2020).
- Hegde, R. S., Zavodszky, E. Recognition and Degradation of Mislocalized Proteins in Health and Disease. Cold Spring Harbor Perspectives in Biology. 11, 033902 (2019).
- Shao, S., Hegde, R. S. A calmodulin-dependent translocation pathway for small secretory proteins. Cell. 147, 1576-1588 (2011).
- Samuelson, J. C., et al. YidC mediates membrane protein insertion in bacteria. Nature. 406, 637-641 (2000).
- Anghel, S. A., McGilvray, P. T., Hegde, R. S., Keenan, R. J. Identification of Oxa1 Homologs Operating in the Eukaryotic Endoplasmic Reticulum. Cell Reports. 21, 3708-3716 (2017).
- Aviram, N., et al. The SND proteins constitute an alternative targeting route to the endoplasmic reticulum. Nature. 540, 134-138 (2016).
- Voorhees, R. M., Hegde, R. S. Structure of the Sec61 channel opened by a signal sequence. Science. 351, 88-91 (2016).
- Cichocki, B. A., Krumpe, K., Vitali, D. G., Rapaport, D. Pex19 is involved in importing dually targeted tail-anchored proteins to both mitochondria and peroxisomes. Traffic. 19, 770-785 (2018).
- Mateja, A., et al. Protein targeting. Structure of the Get3 targeting factor in complex with its membrane protein cargo. Science. 347, 1152-1155 (2015).
- Chacinska, A., Koehler, C. M., Milenkovic, D., Lithgow, T., Pfanner, N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 138, 628-644 (2009).
- Chitwood, P. J., Hegde, R. S. An intramembrane chaperone complex facilitates membrane protein biogenesis. Nature. , (2020).
- Chitwood, P. J., Juszkiewicz, S., Guna, A., Shao, S., Hegde, R. S. EMC Is Required to Initiate Accurate Membrane Protein Topogenesis. Cell. 175, 1-30 (2018).
- Bock, F. J., Tait, S. W. G. Mitochondria as multifaceted regulators of cell death. Nature Reviews Molecular Cell Biology. 21, 85-100 (2020).
- Pfanner, N., Warscheid, B., Wiedemann, N. Mitochondrial proteins: from biogenesis to functional networks. Nature Reviews Molecular Cell Biology. 20, (2019).
- Bykov, Y. S., Rapaport, D., Herrmann, J. M., Schuldiner, M. Cytosolic Events in the Biogenesis of Mitochondrial Proteins. Trends in Biochemical Sciences. 45, 650-667 (2020).
- Pfanner, N., Warscheid, B., Wiedemann, N. Mitochondrial proteins: from biogenesis to functional networks. Nature Reviews Molecular Cell Biology. 427, 1135 (2019).
- Borgese, N., Coy-Vergara, J., Colombo, S. F., Schwappach, B. The Ways of Tails: the GET Pathway and more. The Protein Journal. , 1-17 (2019).
- Mateja, A., Keenan, R. J. A structural perspective on tail-anchored protein biogenesis by the GET pathway. Current Opinion in Structural Biology. 51, 195-202 (2018).
- Chio, U. S., Cho, H., Shan, S. Mechanisms of Tail-Anchored Membrane Protein Targeting and Insertion. Annual review of cell and developmental biology. 33, 417-438 (2017).
- Denic, V. A portrait of the GET pathway as a surprisingly complicated young man. Trends in biochemical sciences. , (2012).
- Hegde, R. S., Keenan, R. J. Tail-anchored membrane protein insertion into the endoplasmic reticulum. Nature Reviews Molecular Cell Biology. 12, 787-798 (2011).
- Kalbfleisch, T., Cambon, A., Wattenberg, B. W. A bioinformatics approach to identifying tail-anchored proteins in the human genome. Traffic. 8, 1687-1694 (2007).
- Doan, K. N., et al. The Mitochondrial Import Complex MIM Functions as Main Translocase for α-Helical Outer Membrane Proteins. Cell Reports. 31, (2020).
- McDowell, M. A., et al. Structural Basis of Tail-Anchored Membrane Protein Biogenesis by the GET Insertase Complex. Molecular Cell. 80, (2020).
- Guna, A., Volkmar, N., Christianson, J. C., Hegde, R. S. The ER membrane protein complex is a transmembrane domain insertase. Science. 591, 3099 (2017).
- Rao, M., et al. Multiple selection filters ensure accurate tail-anchored membrane protein targeting. eLife. 5, 21301 (2016).
- Schuldiner, M., et al. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell. 134, 634-645 (2008).
- Chen, Y. -. C., et al. Msp1/ATAD1 maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. The EMBO journal. 33, 1548-1564 (2014).
- Wu, X., Rapoport, T. A. Translocation of Proteins through a Distorted Lipid Bilayer. Trends in Cell Biology. , (2021).
- Phillips, B. P., Gomez-Navarro, N., Miller, E. A. Protein quality control in the endoplasmic reticulum. Current Opinion in Cell Biology. 65, 96-102 (2020).
- van de Weijer, M. L., et al. Quality Control of ER Membrane Proteins by the RNF185/Membralin Ubiquitin Ligase Complex. Molecular Cell. 79, (2020).
- Weir, N. R., Kamber, R. A., Martenson, J. S., Denic, V. The AAA protein Msp1 mediates clearance of excess tail-anchored proteins from the peroxisomal membrane. eLife. 6, 28507 (2017).
- Gardner, B. M., et al. The peroxisomal AAA-ATPase Pex1/Pex6 unfolds substrates by processive threading. Nature communications. 9, 135 (2018).
- Puchades, C., et al. Unique Structural Features of the Mitochondrial AAA+ Protease AFG3L2 Reveal the Molecular Basis for Activity in Health and Disease. Molecular Cell. , (2019).
- Castanzo, D. T., LaFrance, B., Martin, A. The AAA+ ATPase Msp1 is a processive protein translocase with robust unfoldase activity. Proceedings of the National Academy of Sciences of the United States of America. 117, 14970-14977 (2020).
- Wang, L., Myasnikov, A., Pan, X., Walter, P. Structure of the AAA protein Msp1 reveals mechanism of mislocalized membrane protein extraction. eLife. 9, (2020).
- Puchades, C., Sandate, C. R., Lander, G. C. The molecular principles governing the activity and functional diversity of AAA+ proteins. Nature Reviews Molecular Cell Biology. , 1-16 (2019).
- Yang, Y., et al. Folding-Degradation Relationship of a Membrane Protein Mediated by the Universally Conserved ATP-Dependent Protease FtsH. Journal of the American Chemical Society. , 10 (2018).
- Baldridge, R. D., Rapoport, T. A. Autoubiquitination of the Hrd1 Ligase Triggers Protein Retrotranslocation in ERAD. Cell. 166, 394-407 (2016).
- Fresenius, H. L., Wohlever, M. L. Sorting out how Msp1 maintains mitochondrial membrane proteostasis. Mitochondrion. 49, 128-134 (2019).
- Wang, L., Walter, P. Msp1/ATAD1 in Protein Quality Control and Regulation of Synaptic Activities. Annual Review of Cell and Developmental Biology. 36, 1-24 (2020).
- Dederer, V., et al. Cooperation of mitochondrial and ER factors in quality control of tail-anchored proteins. eLife. 8, 1126 (2019).
- Matsumoto, S., et al. Msp1 Clears Mistargeted Proteins by Facilitating Their Transfer from Mitochondria to the ER. Molecular Cell. , (2019).
- Li, L., Zheng, J., Wu, X., Jiang, H. Mitochondrial AAA-ATPase Msp1 detects mislocalized tail-anchored proteins through a dual-recognition mechanism. EMBO Reports. 20, (2019).
- Weidberg, H., Amon, A. MitoCPR - a surveillance pathway that protects mitochondria in response to protein import stress. Science. 360, (2018).
- Okreglak, V., Walter, P. The conserved AAA-ATPase Msp1 confers organelle specificity to tail-anchored proteins. Proceedings of the National Academy of Sciences of the United States of America. 111, (2014).
- Piard, J., et al. A homozygous ATAD1 mutation impairs postsynaptic AMPA receptor trafficking and causes a lethal encephalopathy. Brain. , (2018).
- Zhang, J., et al. The AAA+ ATPase Thorase regulates AMPA receptor-dependent synaptic plasticity and behavior. Cell. 145, 284-299 (2011).
- Prendergast, J., et al. Ganglioside regulation of AMPA receptor trafficking. The Journal of Neuroscience. 34, 13246-13258 (2014).
- Umanah, G. K. E., et al. Thorase variants are associated with defects in glutamatergic neurotransmission that can be rescued by Perampanel. Science Translational Medicine. 9, 4985 (2017).
- Pignatelli, M., et al. Synaptic Plasticity onto Dopamine Neurons Shapes Fear Learning. Neuron. 93, 425-440 (2017).
- Zhang, J., et al. The AAA Thorase is neuroprotective against ischemic injury. Journal of Cerebral Blood Flow and Metabolism. , 271678 (2018).
- Umanah, G. K. E., et al. AMPA Receptor Surface Expression Is Regulated by S-Nitrosylation of Thorase and Transnitrosylation of NSF. Cell Reports. 33, 108329 (2020).
- Wohlever, M. L., Mateja, A., McGilvray, P. T., Day, K. J., Keenan, R. J. Msp1 Is a Membrane Protein Dislocase for Tail-Anchored Proteins. Molecular Cell. 67, 194-202 (2017).
- Lovell, J. F., et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 135, 1074-1084 (2008).
- Leshchiner, E. S., Braun, C. R., Bird, G. H., Walensky, L. D. Direct activation of full-length proapoptotic BAK. Proceedings of the National Academy of Sciences of the United States of America. 110, 986-995 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved