Method Article
Profiling Sensitivity to Targeted Therapies in EGFR-Mutant NSCLC Patient-Derived Organoids
In This Article
Summary
This protocol describes a standardized evaluation of drug sensitivities to targeted signaling inhibitors in NSCLC patient-derived organoid models.
Abstract
Novel 3D cancer organoid cultures derived from clinical patient specimens represent an important model system to evaluate intratumor heterogeneity and treatment response to targeted inhibitors in cancer. Pioneering work in gastrointestinal and pancreatic cancers has highlighted the promise of patient-derived organoids (PDOs) as a patient-proximate culture system, with an increasing number of models emerging. Similarly, work in other cancer types has focused on establishing organoid models and optimizing culture protocols. Notably, 3D cancer organoid models maintain the genetic complexity of original tumor specimens and thus translate tumor-derived sequencing data into treatment with genetically informed targeted therapies in an experimental setting. Further, PDOs might foster the evaluation of rational combination treatments to overcome resistance-associated adaptation of tumors in the future. The latter focuses on intense research efforts in non-small-cell lung cancer (NSCLC), as resistance development ultimately limits the treatment success of targeted inhibitors. An early assessment of therapeutically targetable mechanisms using NSCLC PDOs could help inform rational combination treatments. This manuscript describes a standardized protocol for the cell culture plate-based assessment of drug sensitivities to targeted inhibitors in NSCLC-derived 3D PDOs, with potential adaptability to combinational treatments and other treatment modalities.
Introduction
Personalized therapies against oncogenic drivers have revolutionized cancer treatment, improving patient survival and reducing treatment-mediated side effects1. Recent advances in molecular diagnostics and sequencing technologies have highlighted the complexity of human tumors, with spatial and temporal heterogeneity impacting treatment response2. Recapitulating these subclonal differences in cell culture models has long been limited to investigating selected alterations of interest in otherwise uniform cell lines. Newly developed 3D PDO models generated from tumor biopsies or surgical tumor resections allow for improved representation of cellular complexity and signaling crosstalk within patient-derived tumor tissue3. As such, tumor organoids derived from gastrointestinal and pancreatic cancer have successfully been generated and recapitulate the genetic diversity and determinants of treatment response4,5,6. In non-small cell lung cancer (NSCLC), organoid development and establishment challenges are acknowledged, and optimization of culture techniques and selective media factors is needed to enable broader and more systematic use of NSCLC PDOs in the future7,8.
Developing combinatorial therapies targeting residual tumor cells that withstand initial drug treatment is essential to inhibit resistance development and ultimately to improve patient survival9. Given the architectural complexity of organoid cultures, classical drug response parameters need to be optimized to allow for accurate and reproducible testing of drug sensitivities. Imaging-based readouts10,11 and classical cell viability assays measuring cellular ATP abundance6,12, amongst other techniques, are available to profile drug responses in PDO cultures. Here, we develop and describe a standardized protocol to evaluate drug sensitivities to targeted therapy against known clinical drivers in NSCLC PDO models.
Protocol
For human subjects research, informed consent was obtained and tissue collection was carried out under the UCSF Internal Review Board approved protocols (IRB, protocol no.: #13-12492, or CC#17-23309). The establishment of organoid cultures from de-identified clinical specimens was performed in collaboration with research partners according to previously published methods13,14,15,16. Organoid cultures were retrieved for maintenance and drug escalation experiments at passage three or later. All the following protocols were performed under aseptic conditions in a mammalian tissue culture laboratory environment.
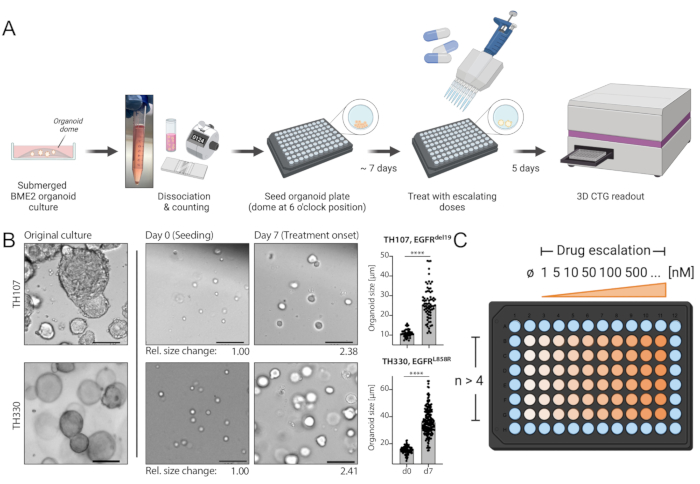
Figure 1: Protocol schematic of workflow and critical steps in the technique. (A) Experimental workflow including seeding of organoids in 96-well format, treatment with drug escalation at 7 days after seeding, and luminescence-based cell survival readout 5 days after treatment using an ELISA plate reader. (B) An example image of the EGFRdel19-positive TH107 and EGFRL858R-positive TH330 NSCLC organoid cultures. Original cultures, cells at the time of seeding (day 0), and organoids at treatment start 7 days after seeding (day 7) are shown. Scale bar = 100 µm. Changes in organoid diameter over the initial 7-day culture period are quantified and indicate >2-fold increase in organoid size. Relative fold changes in sizes at day 7 compared to average size at day 0 are presented below the representative images. For TH107, a fold change of 2.38 over 7 days is observed, indicating a doubling time of 5.88 days (141.12 h). For TH330, a fold change of 2.41 over 7 days is observed, indicating a doubling time of 5.81 days (139.42 h). Quantification of changes in organoid size and statistical evaluation are presented (right). Statistical significance is calculated by unpaired t-test, p < 0.0001. (C) Treatment layout for drug escalation in organoid 96-well plate format. The number of technical replicates and exemplary doses are indicated, including a negative control. The schematics are created with BioRender, a web-based illustration tool. Please click here to view a larger version of this figure.
1. Experimental preparations
- Prepare growth medium (GM) as previously reported15: Dulbecco's Modified Eagle's Medium/Ham's nutrient mixture F12 (DMEM/F-12) with L-alanyl-L-glutamine, supplemented with 100 U/mL penicillin/streptomycin, 10 mM HEPES, 25 nM hRspondin, 1x B27, 5 mM Nicotinamide, 1.25 mM N-Acetylcysteine, 500 nM A-8301, 500 nM SB202190, 50 µg/mL Primocin, 100 ng/mL hNoggin, 100 ng/mL hFGF-10, 25 ng/mL hFGF-7 (see Table of Materials).
NOTE: Mix gently to avoid foaming and filter through a 0.22 µm filtering system. Warm media to 37 °C within 1 h before use. - Prepare low growth factor media (LGM) as previously reported16 without the addition of epidermal growth factor (EGF): Advanced Dulbecco's Modified Eagle's Medium/Ham's nutrient mixture F12 (DMEM/F-12), supplemented with 1 mM HEPES, 1x L-alanyl-L-glutamine, 1x Penicillin-Streptomycin-Glutamine, 10 mM Nicotinamide, 1 mM N-Acetylcysteine, 1x B27, 500 nM A-8301, 100 ng/mL hNoggin.
NOTE: Mix gently to avoid foaming and filter through a 0.22 µm filter. Warm media to 37 °C within 1 h before use. - Thaw BME2 (Reduced Growth Factor Basement Membrane Extract, Type 2, see Table of Materials) on ice, at 4 °C, overnight.
2. Generating single-cell suspension and seeding of cells
- Dissociate submerged BME2 organoid culture as described in the following steps (2.1.1-2.1.6).
- Carefully aspirate media from the culture plates. Avoid touching the submerged BME2 organoid culture.
NOTE: Media Aspiration can be done as the researcher prefers, e.g., with a basic fluid aspiration system or using a pipette. Avoid touching the BME2 embedded organoids as this might result in loss of organoid biomass. - Trypsinize the submerged BME2 organoid culture with a suitable recombinant enzyme (see Table of Materials). In 6-well plate format, add 2 mL per well. Mechanically break the BME2 by repeatedly pipetting up and down. Incubate plates at 37 °C in the cell culture incubator for 5 min.
- Transfer the suspension into a 15 mL centrifuge tube and centrifuge at 600 x g for 5 min at room temperature.
- Aspirate the recombinant enzyme carefully without touching the organoid pellet.
NOTE: Residual BME2 may be present. Repeat the enzyme digestion if needed. - Resuspend the organoid pellet in GM (step 1.1). Add DNase I 1x 100 U/mL and incubate for 5 min at room temperature (see Table of Materials).
- Centrifuge at 600 x g for 3 min at room temperature. Pipette off the media carefully without touching the organoid pellet and discard. Resuspend in fresh GM.
- Carefully aspirate media from the culture plates. Avoid touching the submerged BME2 organoid culture.
- Seeding of organoid single-cell suspension
- Pre-heat a new black, clear-bottom 96-well plate at 37 °C in the cell culture incubator for 10 min.
NOTE: Using clear bottom plates is essential to monitor organoid growth and drug response. - For counting, prepare a 1:5 dilution of the cell suspension in PBS (total volume: 500 µL) and count the cell suspension using a cell analyzer (see Table of Materials).
NOTE: Make sure to multiply by the dilution factor (x 5) to receive the final cell concentration. Alternative counting methods such as a hemocytometer can be used. Cell viability should be monitored using a viability staining assay (e.g., with Trypan Blue17). Using a cell analyzer, viability is assessed automatically. The viability of organoid single-cell suspensions as evaluated by the cell analyzer should be ≥95% (Supplementary Figure 1). - Calculate the volume of the cell suspension needed to seed for the experiment.
NOTE: The seeding concentration is 1500 cells/µL BME2, with 5 µL BME2 needed per well (Total number: 7500 cells/well). For one 96-well plate, 6 x 10E5 cells are required. This includes the seeding of 60 wells with organoid domes (4.5 x 10E5) and experimental surplus (calculation for a total of 80 wells). - Prepare one aliquot of cell suspension in a 1.5 mL microcentrifuge tube per each 96-well plate planned to be seeded if multiple 96-well plates are included in the experiment.
NOTE: Experimental surplus and separate aliquots per seeded 96-well plate are needed to account for the increased experimental bias due to handling BME2. - Aliquot cells from the single-cell suspension as calculated (step 2.2.3) after careful resuspension by pipetting up and down. Pellet cells at 600 x g for 5 min at room temperature. Carefully remove media using a P200 pipette without touching the cell pellet. Place cell pellet on ice shortly (~1 min) and resuspend the cell pellet in BME2.
NOTE: Residual media may compromise BME2 structure and rigidity. Pipette off all media carefully. Place cells on ice shortly to acclimate the cell pellet and allow cells to be resuspended in BME2 without clumping. Keep BME2 on ice constantly for it to stay in the liquid state. For one 96-well plate, 400 µL BME2 is needed for resuspension of the cell pellet. Resuspend cell pellets carefully, avoiding the introduction of bubbles. - Tilt your pre-warmed black, clear-bottom 96-well plate towards you. Plate cells using 5 µL of cell suspension per well and seeding cell domes at the 6 o'clock position of each well (Figure 1A). Seed the cell domes in the remaining inner wells (columns 2-10 and rows B-G of a 96-well plate).
NOTE: Reverse pipetting18 is recommended when handling BME2. - Do not move the 96-well plate and incubate freshly seeded cell domes in the cell culture laminar flow hood for 5 min at room temperature. Then move the plate to the cell culture incubator and incubate it for 10 min at 37 °C.
- Carefully add 100 µL of GM per well to all the wells containing organoids (columns 2-10 and rows B-G of a 96-well plate). Add 100 µL of PBS to the outer wells at the rim of the plate.
- Culture BME2 embedded organoids in GM media at 37 °C in the cell culture incubator for a total of 7 days. Inspect growth of organoids under the light microscope regularly.
NOTE: Please refer to Figure 1B and Supplementary Table 1 for an example of expected growth progress from seeding to the day of treatment. - Change the media once after 3-4 days of culture: rotate the plate clockwise by 180° (organoids now at 12 o'clock position), carefully aspirate GM from opposite position to organoid dome using a multichannel apparatus if available, and then add fresh GM.
- Pre-heat a new black, clear-bottom 96-well plate at 37 °C in the cell culture incubator for 10 min.
3. Drug treatment
- Prepare a serial drug dilution in LGM for the drug of choice, e.g., osimertinib, to treat EGFR-mutant NSCLC organoids. Include a negative control (LGM media + 0.1% DMSO). Prepare sufficient drug aliquots of all doses according to the number of wells seeded plus experimental surplus.
NOTE: A dilution series including ≥8 doses and ranging from 1 nM-10 µM is recommended for targeted inhibitors. - Rotate the organoid plate clockwise by 180° (organoids now at 12 o'clock position). Carefully aspirate GM using a multichannel apparatus preferably.
NOTE: Avoid touching the organoid dome while aspirating the GM as this may result in loss of organoid biomass and impact results. - Add 100 µL of control (e.g., LGM media + 0.1% DMSO) or drug solution per well.
NOTE: Please refer to Figure 1C for the drug escalation treatment schematic in 96-well plate format. - Incubate treated organoids at 37 °C in the cell culture incubator for 5 days.
4. Readout by luminescence-based survival assay
- Harvest and survival readout
- Perform the survival assay according to Reference19.
- Thaw reagent (see Table of Materials) overnight at 4 °C. Equilibrate reagent in a water bath at room temperature for 30 min before use and mix by inverting.
- Add an equal volume of the reagent to each well (100 µL per well). Mix thoroughly by pipetting up and down, with the pipette tip placed at the position of the organoid dome. Incubate for 5 min at room temperature in the dark.
- Using a multichannel pipette, transfer approximately 75% (150 µL) of the lysate (step 4.1.1.2) to a new white, opaque-bottom 96-well plate.
NOTE: Transferring 75% of lysates to a new plate ensures the absence of bubbles in the later readout without impacting assay sensitivity. - Incubate for an additional 25 min at room temperature in the dark.
- Record luminescence using an ELISA plate reader (integration time 0.25-1 s/per well) (see Table of Materials).
- Save data in an appropriate format, e.g., a data table containing all raw reads and recording of the plate layout and drugs used.
- Perform the survival assay according to Reference19.
- Data analysis using statistical analysis software (see Table of Materials)
- Create a new XY-table and insert data in an XY format: Rows (X) are negative control followed by escalating drug doses, with doses as log [Inhibitor] in Molar concentration. Columns (Y) are readout values that include replicates stacked along with columns.
NOTE: The concentration of the negative control should be indicated as a minimal value (given 0 is not possible in log scale), e.g., log [Inhibitor], M = -10. - Normalize values by selecting Analyze > Normalize and using the following parameters: normalize each subcolumn separately, Y = 0 as 0 %, "last value in each subcolumn (or first, whichever is larger)" as 100 %, results in percentages, graph the results.
- Fit non-linear regression curve on normalized data by selecting Analyze > XY analyses > Non-linear regression > Dose response - Inhibition > log (inhibitor) vs . normalized response -- Variable slope.
- Report results as a table of IC50 values outputted after non-linear regression analysis and graph of response curve including normalized data points as mean +/- standard deviation and fitted regression curve.
- Create a new XY-table and insert data in an XY format: Rows (X) are negative control followed by escalating drug doses, with doses as log [Inhibitor] in Molar concentration. Columns (Y) are readout values that include replicates stacked along with columns.
Results
Considerable challenges in establishing NSCLC organoids have been noted7. Thus, it is exciting to see recent work establishing lung cancer organoids and using them for drug treatment assays20,21,22. EGFR-mutations account for 11.3% of NSCLC cases23. Targeted treatment with EGFR inhibitors represents the first-line treatment option in EGFR-mutant NSCLC and has improved the overall survival and treatment safety in patients24. This work determined the sensitivity to the FDA-approved EGFR tyrosine kinase inhibitor osimertinib24,25 in EGFR-mutant NSCLC organoids. EGFR-mutant NSCLC organoids were generated from surgical resection or tumor biopsy specimens of NSCLC patients and confirmed to harbor the indicated oncogenic mutation by DNA sequencing. As outlined above, EGFR-mutant NSCLC organoid models were treated with escalating doses of osimertinib and PDO viability assessed by luminescence-based cell survival readout five days after the treatment initiation. While EGFR mutant (EGFRdel19)-positive TH107 organoids showed sensitivity to osimertinib treatment with a half-maximal inhibitory concentration (IC50) of 56 nM (Figure 2A), EGFR-mutant (EGFRL858R)-positive TH116 organoids were resistant to osimertinib treatment with an IC50 of greater than 1 µM (Figure 2B). The sensitivity of EGFRdel19-positive TH107 NSCLC was accompanied by significant transcriptional changes, including a reduction in the expression of cell cycle-associated gene signatures and an increase in the expression of apoptosis-associated gene signatures (Supplementary Figure 2A,B). As a reference, response data for the sensitive EGFRdel19-positive NSCLC cell line PC9 is presented (Figure 3A,B). The latter includes survival analysis to escalating doses of osimertinib by a 2D luminescence-based survival assay (Figure 3A) and the study of signaling suppression on the level of EGFR-MAPK signaling by Western blot (Figure 3B). Overall, this data highlights the accuracy of the present protocol for determining drug response and distinguishing between sensitive and resistant NSCLC PDO models. Further analyses of EGFRL858R-positive TH116 organoid and available clinical specimens are needed to determine possible resistance-associated alterations.
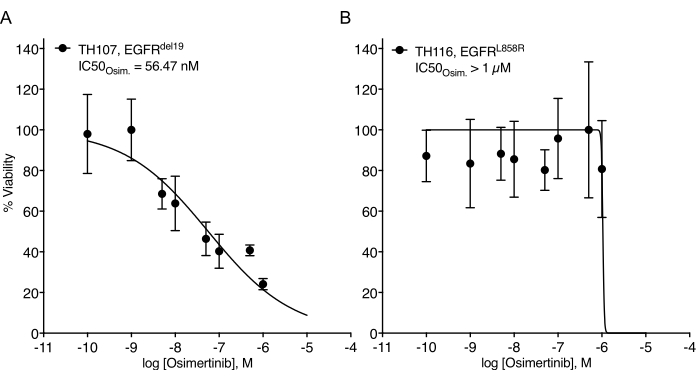
Figure 2: Treatment response curve of EGFR-mutant NSCLC organoid models to osimertinib escalation. (A) Osimertinib response in the sensitive EGFRdel19-positive TH107 NSCLC organoid model. (B) Osimertinib response in the resistant EGFRL858R-positive TH116 NSCLC organoid model. Data points are presented as normalized values showing the mean +/- standard deviation, with a non-linear regression curve fitted through the data. TH107, n = 6 technical replicates per data point. TH116, n = 4 technical replicates per data point. Please click here to view a larger version of this figure.
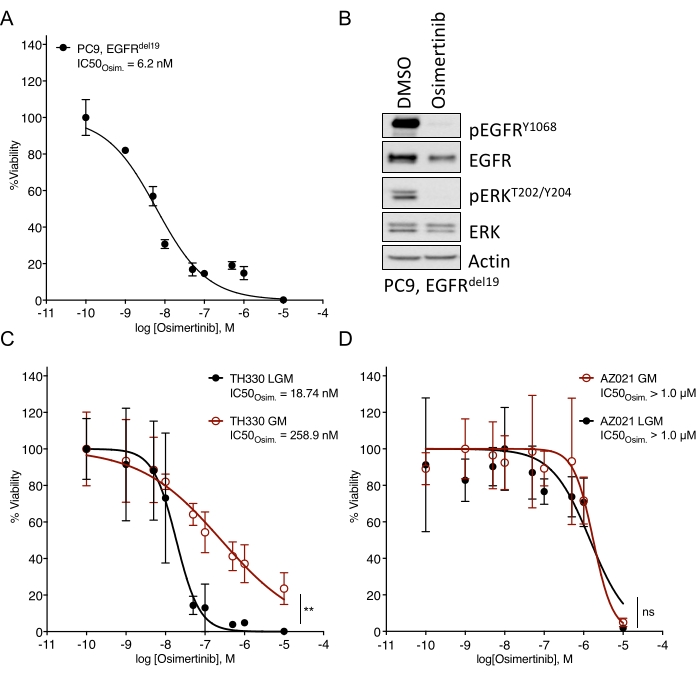
Figure 3: Comparative data for the treatment response to osimertinib in a sensitive EGFR-mutant NSCLC cell line and organoid models cultured in different media. (A) Osimertinib response in the sensitive EGFRdel19-positive NSCLC cell line PC9, determined by standard 2D-CTG assay. (B) Signaling suppression in the PC9 cells upon two-day treatment with osimertinib (2 µM). (C) Osimertinib response in the sensitive EGFRL858R-positive TH330 NSCLC organoid model culture in LGM and GM media. (D) Osimertinib response in the resistant AZ021 NSCLC organoid model in LGM and GM media. Confirmation of the oncogenic EGFRL858R mutation in AZ021 failed and may be causative for the lack of osimertinib response. For A and C-D, data points are presented as normalized values showing the mean +/- standard deviation, with a non-linear regression curve fitted through the data. PC9, n = 3 technical replicates per data point. TH330, n = 5 technical replicates per data point. AZ021, n = 6 technical replicates per data point. A Wilcoxon rank test was performed on normalized data to determine the statistical significance. For TH330 (C), LGM vs. GM, ** p = 0.0078. For AZ021 (D), LGM vs. GM, ns p = 0.0742. Please click here to view a larger version of this figure.
Supplementary Figure 1: Representative cell analyzer results for counting and viability assessment of EGFR-mutant organoid models. TH107 and TH107BC refer to different organoid models and A and B to biological replicates. For each model and biological replicate, three technical replicates are counted; all show ≥95% viability. A representative image during cell counting is presented on the right, showing robust viability and single-cell dissociation. Please click here to download this File.
Supplementary Figure 2: Gene set enrichment analysis (GSEA) using bulk RNA sequencing data obtained for EGFR-mutant TH107 NSCLC organoids, comparing untreated control (DMSO) and cells treated with Osimertinib for 3 days (OSI_D3). (A-B) Upon targeted treatment, sensitive cells will undergo cell cycle G1 arrest and cease active proliferation. As an expression of G2M cell cycle genes is associated with active proliferation, a nominal enrichment of expression in the untreated control (DMSO) is expected. For apoptosis-related genes, a nominal enrichment in treated cells (OSI_D3) is expected. Both have been confirmed in the EGFR-mutant TH107 NSCLC organoid model treated with Osimertinib: (A) GSEA for Hallmark G2M expression signature (left) shows enrichment in DMSO- treated cells. Nominal enrichment score (NES): +1.708, FDR < 0.0001. (B) GSEA for Hallmark Apoptosis expression signature (right) shows enrichment in Osimertinib-treated cells. NES: -1.075, FDR: ns, 0.3275. Please click here to download this File.
Supplementary Figure 3: Combinatorial drug treatment in EGFR-mutant TH330 organoid model treated with Osimertinib escalation in the presence of a second additive inhibitor at a fixed concentration. Resistance-associated alterations in EGFR-mutant NSCLC, i.e., SRC and AXL activation26,27,28, were pharmacological targeted by combinatorial treatment with SRC inhibitor Saracatinib (100 nM) or AXL inhibitor R428 (500 nM), n = 6 technical replicates per data point. Both combinatorial treatments resulted in increased treatment response, with significance for the combination of Osimertinib with SRC inhibitor Saracatinib. Statistical significance was evaluated by Wilcoxon rank test: Osimertinib versus Osimertinib + Saracatinib, *p = 0.0195; Osimertinib vs. Osimertinib + R428, ns, p = 0.2500. Please click here to download this File.
Supplementary Table 1: Change of organoid size from seeding (d0) to 7 days post-treatment (d7). (A) Growth development in EGFRdel19-positive NSCLC organoid TH107. (B) Growth development in EGFRL858R-positive NSCLC organoid TH330. Please click here to download this Table.
Discussion
This manuscript develops and describes a standardized protocol for assessing drug sensitivity in NSCLC-derived 3D PDO models. In addition to drug sensitivity studies, further characterization of available organoid models is needed to determine the underlying causes for differences in drug sensitivity. This may include genetic profiling of organoids and patient specimens and other analysis available for organoids, such as immunohistochemistry staining for differentiation markers and general cellular signaling biomarkers and physiology13,29.
Critical steps in the protocol
The protocol outlined herein provides a standardized workflow that allows accurate and reproducible drug sensitivity analyses when followed carefully. Particular care should be taken in the following steps: TrypLE and DNAse I digestion during generation of single-cell suspensions, seeding of single-cell suspensions in BME2, monitoring organoid growth until treatment, media changes, and disruption and lysis of BME2 embedded organoids during luminescence-based cell survival readout. (1) While additional DNAse I digestion after TrypLE-based dissociation of organoids is not essential for expanding organoid models during regular culture maintenance, DNAse I digestion should not be omitted when seeding for drug escalation experiments as it ensures better separation of organoid clusters into single-cell suspensions and accurate cell counting. (2) Seeding of single-cell suspension in BME2 represents a critical step given the solidification of BME2 at room temperature. Thus, a maximum of 1-2 rows needs to be seeded at once, and samples should be placed on ice before additional rows are seeded. Of note, cells need to be pipetted up and down when seeding is continued to allow for a homogeneous cell suspension. (3) Organoid growth needs to be monitored carefully during the 7-day expansion from seeding to treatment. An example of the expected development is given in Figure 1B and Supplementary Table 1. Of note, assessing changes in organoid size by brightfield microscopy and image analysis as presented in Figure 1B may allow for a precise evaluation of differences in organoid growth and doubling times. Doubling times can have an impact on drug responses, as recently discussed in the literature30. If the organoid growth rate exceeds the presented example significantly, a shorter expansion time until the start of treatment and shorter treatment duration can be considered. (4) In addition, special care should be taken when changing media to avoid aspirating organoids. The seeding position of BME2 embedded organoids at the 6 o'clock position allows for a safe aspiration of media when plates are turned clockwise by 180° and media is aspirated at the opposite position of the organoids. (5) Finally, thorough lysis of BME2 embedded organoids during the survival readout is essential to record accurate results. According to the manufacturer's instructions, samples should be pipetted up and down repeatedly, ideally using unfiltered tips, to ensure proper lysis. Incubation times should be followed as described. Further, transferring 75% of the lysate (instead of the total volume) to a white, opaque-bottom 96-well plate for the final readout using an ELISA plate reader allows for an appropriate assessment, as this assures the same volume in each well and the absence of air bubbles that can be introduced by vigorous pipetting.
Of note, profiling of drug responses in BME2-embedded organoid cultures may show a higher standard deviation than observed in regular cell line cultures (Figure 2, Figure 3A). The higher standard deviation is based on several factors, including an increased likelihood of minor variations in seeding when working with BME2 and differences in individual organoid growth rates across wells over the initial 7-day growth period. Thus, equal or more than four technical replicates per drug concentration should be seeded.
Most importantly, the presence of malignant cells carrying the oncogenic driver mutation and limited contamination by normal airway epithelial cells must be carefully evaluated. Challenges in NSCLC establishment can favor the outgrowth of normal airway epithelial cells7. Copy number profiling or PCR- and sequencing-based approaches to confirm the presence of the oncogenic driver mutations are the methods of choice to ensure the quality of NSCLC organoid cultures.
Modifications and troubleshooting of the method
Media and respective growth factors added to basic media solutions can significantly impact drug response to targeted inhibitors. They activate bypass receptors and signaling pathways that influence and limit drug response (e.g., FGF, HGF, EGF)26. While a growth-factor rich and tailored media may be optimal for expanding organoid culture, drug escalation and sensitivity assessments should be performed in a reduced growth-factor media, as outlined above. This is based on internal experience comparing different media formulations and drug response data (Figure 3C). While media solutions can affect the degree of sensitivity to certain drug treatment and can shift IC50 values, robust phenotypes of sensitivity or resistance are apparent irrespective of media formulation (Figure 3C,D). In addition, general consistency in the media formulation and profiling drug responses across organoid cultures is recommended, and an equal or more than four technical replicates per concentration needs to be seeded. This is particularly important to benchmark ranges in sensitivity vs. resistance for the Inhibitor of interest.
Limitations of the method
The protocol presented here describes the sensitivity of NSCLC 3D cancer organoid models to targeted inhibitors when patient-derived cancer cells are cultured. Additional experiments, including pharmacodynamic analysis regarding pathway inhibition and sequencing analysis for the presence of the driver oncogene and secondary mutations, are needed for a detailed characterization of drug resistance and sensitivity. Further, bystander factors such as microenvironmental stimuli derived from interactions or secreted factors by non-cancer bystander cells in the tumor microenvironment are not accounted for, and novel protocols are needed when co-culture organoid models with immune or stromal cells are attempted. Recent work has highlighted the use of organoid models to recapitulate tumor microenvironment interactions and profile responses to immune checkpoint inhibitors, such as anti-PD-L1 treatment13,31.
The significance of the method with respect to existing / alternative methods
3D cancer organoid models recapitulate the genetic diversity and determinants of treatment response present in the original tumor4,5,6. Notably, spatial and temporal heterogeneity can promote tumor evolution, and parallel emergence and the sequential development of tumor subclones can occur32,33. Intratumor heterogeneity is significant for the selection of more resilient tumor cells under therapeutic pressure9,34,35. The protocol provided here allows for a rapid assessment of sensitivities to treatment with targeted inhibitors in patient-proximate samples. Thus, organoid models have advantages over more conventional homogeneous cell line models lacking genetic diversity or long-term studies using cell lines or patient-derived xenografts. Further, the present protocol allows scaling up to multiple arms of treatment and combinational treatment approaches with few limitations regarding cost and analytic capacity. As such, adding a second drug of interest at a fixed dose while escalating the primarily targeted Inhibitor and comparing it to the escalation of the primarily targeted Inhibitor alone allows for efficiently evaluating the potential combinatorial effects and with minimal additional biomass required (Supplementary Figure 3). Compared to imaging-based assessments used to monitor organoid development and drug response, the luminescence-based cell survival assay described here has similar sensitivity with minimal equipment and training required.
Importance and potential applications of the method in specific research areas
Developing a standardized pipeline that allows for establishing cancer organoid models from patient specimens and the subsequent drug sensitivities profiling holds significant clinical applicability potential. Ex vivo pharmacological profiling has gained recognition in detecting vulnerabilities and resistant-associated features in tumors, correlating to treatment response in patients36,37. Significantly, ex vivo profiling of drug sensitivities may aid in treatment selection in the clinic and the design of rational combinational treatments addressing resistance mechanisms. Overall, this approach could help to enable improved personalized strategies for molecular therapy or combinatorial treatment regimens. The latter may help target drug tolerance and resistance mechanisms early and deepen clinical response to improve patient outcomes in the future.
Disclosures
T.G.B. is an advisor to Array Biopharma, Revolution Medicines, Novartis, AstraZeneca, Takeda, Springworks, Jazz Pharmaceuticals, Relay Therapeutics, Rain Therapeutics, Engine Biosciences, and receives research funding from Novartis, Strategia, Kinnate, and Revolution Medicines.
Acknowledgements
We thank the laboratories of Jeroen P Roose (UCSF) and Calvin J Kuo (Stanford) for their input regarding organoid culture and protocol development. We further thank Oghenekevwe M. Gbenedio (Roose lab, UCSF) for protocols and sample establishment input. This research project was conducted with support from the NIH [U54CA224081]. F. Haderk was supported by the Mildred Scheel postdoctoral fellowship from the German Cancer Aid.
Materials
| Name | Company | Catalog Number | Comments |
| 1.5 mL tubes | |||
| 15 mL centrifuge tubes | |||
| 500 mL Vacuum Filter/Storage Bottle System, 0.2 µm Pore 33.2 cm2 Nylon Membrane | Corning | 430773 | for both media |
| 96-Well, Cell Culture-Treated, Flat Clear Bottom Black Microplate | Corning | 3904 | |
| 96-Well, Cell Culture-Treated, Solid White Flat-Bottom Microplate | Corning | 3917 | |
| A-8301 | Tocris Bioscience | 293910 | for both media |
| Advanced DMEM/F-12 | Gibco | 12634010 | for LGM |
| B27 | Life Technologies | 12587010 | for both media |
| BioRender 2021 | https://biorender.com/ | online scientific illustration software | |
| BME2 (Cultrex RGF Basement Membrane Extract, Type 2) | R&D Systems | 353301002 | |
| Cell culture incubator (37 °C, 5% CO2) | |||
| CellTiter-Glo 3D Cell Viability Assay | Promega | G9682 | 3D-CTG readout reagent |
| Centrifuge holding 15 mL centrifuge tubes | |||
| Deoxyribonuclease I (DNAse I) | ThermoFisher Scientific | 18047019 | |
| Dulbecco's Phosphate-Buffered Salt Solution | Corning | MT21031CV | |
| DMEM/F-12, GlutaMAX supplement | Gibco | 10565018 | for GM |
| GlutaMax | Gibco | 35050061 | for LGM |
| GraphPad Prism software (version 9.2.0) | GraphPad | statistical analysis software | |
| HEPES | Gibco | 15630080 | for both media |
| hFGF-10 | PeproTech | 100-26-100ug | for GM |
| hFGF-7 | PeproTech | 100-19-50ug | for GM |
| hNoggin | PeproTech | 120-10C-100ug | for both media |
| hRspondin | PeproTech | 120-38-100ug | for GM |
| Low retention pipette tips, 20 µL (P20) | ThermoFisher Scientific | 2149P-05-HR | |
| Low retention pipette tips, 200 µL (P200) | ThermoFisher Scientific | 2069-05-HR | |
| Regular length pipette tips, 1000 µL (P1000) | ThermoFisher Scientific | 2179-HR | |
| Multichannel pipette | |||
| N-Acetylcysteine | Fisher Scientific | 50-424-777 | for both media |
| Nicotinamide | Sigma Aldrich | N0636-100G | for both media |
| Osimertinib | Selleck Checm | S7297 | |
| Penicillin-Streptomycin-Glutamine | Gibco | 10378016 | for LGM |
| Penicillin/Streptomycin | Cytiva HyClone | SV30010 | for GM |
| Pipettes (different sizes) | |||
| Plate reader | Molecular Devices | SpectraMax M5 | equipment, alternative readers may be used |
| Primocin | Invivogen | ant-pm-1 | for GM |
| SB202190 | Selleck Chem | S1077 | for GM |
| TrypLE Express Enzyme | Gibco | 12604021 | |
| Vacuum pump and tubing | |||
| Vi-CELL XR Cell Analyzer | Beckman Coulter | Vi-CELL XR | cell analyzer / counter |
References
- de Bono, J. S., Ashworth, A. Translating cancer research into targeted therapeutics. Nature. 467 (7315), 543-549 (2010).
- Dagogo-Jack, I., Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nature Reviews Clinical Oncology. 15 (2), 81-94 (2018).
- Drost, J., Clevers, H. Organoids in cancer research. Nature Reviews Cancer. 18 (7), 407-418 (2018).
- Tiriac, H., et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discovery. 8 (9), 1112-1129 (2018).
- Ooft, S. N., et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Science Translational Medicine. 11 (513), (2019).
- Vlachogiannis, G., et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 359 (6378), 920-926 (2018).
- Dijkstra, K. K., et al. Challenges in establishing pure lung cancer organoids limit their utility for personalized medicine. Cell Reports. 31 (5), 107588(2020).
- Lo, Y. -H., Karlsson, K., Kuo, C. J. Applications of organoids for cancer biology and precision medicine. Nature Cancer. 1 (8), 761-773 (2020).
- Bivona, T. G., Doebele, R. C. A framework for understanding and targeting residual disease in oncogene-driven solid cancers. Nature Medicine. 22 (5), 472-478 (2016).
- Jabs, J., et al. Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Molecular Systems Biology. 13 (11), 955(2017).
- Tashiro, T., et al. In vivo and ex vivo cetuximab sensitivity assay using three-dimensional primary culture system to stratify KRAS mutant colorectal cancer. PLoS One. 12 (3), 0174151(2017).
- van de Wetering, M., et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 161 (4), 933-945 (2015).
- Neal, J. T., et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell. 175 (7), 1972-1988 (2018).
- Hysenaj, L., et al. SARS-CoV-2 infection studies in lung organoids identify TSPAN8 as novel mediator. bioRxiv. , (2021).
- Sachs, N., et al. Long-term expanding human airway organoids for disease modeling. The EMBO Journal. 38 (4), 100300(2019).
- Salahudeen, A. A., et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature. 588 (7839), 670-675 (2020).
- Strober, W. Trypan blue exclusion test of cell viability. Current Protocol in Immunology. , Appendix 3, Appendix 3B (2001).
- Pushparaj, P. N. Revisiting the micropipetting techniques in biomedical sciences: A fundamental prerequisite in good laboratory practice. Bioinformation. 16 (1), 8-12 (2020).
- Promega Corporation. CellTiter-Glo 3D Cell Viability Assay. Promega Corporation. , (2021).
- Kim, M., et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nature Communication. 10 (1), 3991(2019).
- Hu, Y., et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nature Communication. 12 (1), 2581(2021).
- Shi, R., et al. Organoid cultures as preclinical models of non-small cell lung cancer. Clinical Cancer Research. 26 (5), 1162-1174 (2020).
- Collisson, E. A., et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 511 (7511), 543-550 (2014).
- Ramalingam, S. S., et al. Overall Survival with Osimertinib in untreated, EGFR-mutated advanced NSCLC. New England Journal of Medicine. 382 (1), 41-50 (2019).
- Soria, J. -C., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. New England Journal of Medicine. 378 (2), 113-125 (2017).
- Rotow, J., Bivona, T. G. Understanding and targeting resistance mechanisms in NSCLC. Nature Reviews Cancer. 17 (11), 637-658 (2017).
- Zhang, Z., et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nature Genetics. 44 (8), 852-860 (2012).
- Kanda, R., et al. Erlotinib resistance in lung cancer cells mediated by integrin β1/Src/Akt-driven bypass signaling. Cancer Research. 73 (20), 6243-6253 (2013).
- Bruun, J., et al. Patient-derived organoids from multiple colorectal cancer liver metastases reveal moderate intra-patient pharmacotranscriptomic heterogeneity. Clinical Cancer Research. 26 (15), 4107-4119 (2020).
- Hafner, M., Niepel, M., Chung, M., Sorger, P. K. Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nature methods. 13 (6), 521-527 (2016).
- Yuki, K., Cheng, N., Nakano, M., Kuo, C. J. Organoid models of tumor immunology. Trends in Immunology. 41 (8), 652-664 (2020).
- Gerlinger, M., et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. New England Journal of Medicine. 366 (10), 883-892 (2012).
- McGranahan, N., Swanton, C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 27 (1), 15-26 (2015).
- Rambow, F., et al. Toward minimal residual disease-directed therapy in melanoma. Cell. 174 (4), 843-855 (2018).
- Marine, J. C., Dawson, S. J., Dawson, M. A. Non-genetic mechanisms of therapeutic resistance in cancer. Nature Reviews Cancer. 20 (12), 743-756 (2020).
- Frismantas, V., et al. Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia. Blood. 129 (11), 26-37 (2017).
- Drusbosky, L. M., et al. Predicting response to BET inhibitors using computational modeling: A BEAT AML project study. Leukemia Research. 77, 42-50 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved