A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Versatility of Protocols for Resistance Training and Assessment Using Static and Dynamic Ladders in Animal Models
In This Article
Summary
The present protocol describes resistance training and testing using static and dynamic ladders in animal models.
Abstract
Resistance training is a physical exercise model with profound benefits for health throughout life. The use of resistance exercise animal models is a way to gain insight into the underlying molecular mechanisms that orchestrate these adaptations. The aim of this article is to describe exercise models and training protocols designed for strength training and evaluation of resistance in animal models and provide examples. In this article, strength training and resistance evaluation are based on ladder climbing activity, using static and dynamic ladders. These devices allow a variety of training models as well as provide precise control of the main variables which determine resistance exercise: volume, load, velocity, and frequency. Furthermore, unlike resistance exercise in humans, this is a forced exercise. Thus, aversive stimuli must be avoided in this intervention to preserve animal welfare. Prior to implementation, a detailed design is necessary, along with an acclimatization and learning period. Acclimatization to training devices, such as ladders, weights, and clinical tape, as well as to the manipulations required, is necessary to avoid exercise rejection and to minimize stress. At the same time, the animals are taught to climb up the ladder, not down, to the resting area on the top of the ladder. Resistance evaluation can characterize physical strength and permit adjusting and quantifying the training load and the response to training. Furthermore, different types of strength can be evaluated. Regarding training programs, with appropriate design and device use, they can be sufficiently versatile to modulate different types of strength. Furthermore, they should be flexible enough to be modified depending on the adaptive and behavioral response of the animals or the presence of injuries. In conclusion, resistance training and assessment using ladders and weights are versatile methods in animal research.
Introduction
Physical exercise is a determinant lifestyle factor for promoting health and decreasing the incidence of the most prevalent chronic diseases as well as some types of cancer in humans1.
Resistance exercise has raised interest because of its overwhelming relevance for health throughout life2, especially due to its benefits in counteracting age-related diseases that affect the locomotor system, such as sarcopenia, osteoporosis, etc3. Moreover, resistance exercise also affects tissues and organs not directly involved in the execution of movement, such as the brain4. This relevance in recent years has encouraged the development of resistance exercise models in animals to study the underlying tissular and molecular mechanisms, when it is not possible in humans or when the animals provide better insight and are a more controlled model.
Unlike resistance exercise in humans, for animal models researchers usually rely on forced procedures. However, aversive stimuli must be avoided in this context, mainly to preserve animal welfare, reduce stress, and decrease the severity of the experimental procedures5. It should be noted that animals enjoy exercise even in the wild6. For these reasons, it is necessary to improve adaptation to the experiment through prolonged stepwise acclimatization.
The devices, materials, and protocols used for resistance training and assessment in experimental animals must allow the precise control and modulation of numerous variables: load, volume, speed, and frequency7. They should also allow different types of muscle contractions to be performed: concentric, eccentric, or isometric. Considering the above, the protocols used should be able to specifically evaluate or train for different applications of strength: maximal strength, hypertrophy, speed, and endurance.
There are several methods of strength training, such as jumping in water8,9, weighted swimming in water10, or muscle electrostimulation11. However, static and dynamic ladders are versatile devices that are widely used12,13,14.
Resistance assessment in experimental animal models provides valuable information for many research settings, such as describing the phenotypic characteristics of genetically modified animals, evaluating the effect of different intervention protocols (dietary components supplementation, drug treatments, microbiota transplantation, etc.), or assessing the effect of training protocols. Training models provide insight into the physiology of adaptation to strength exercise, which helps to better understand the effect of exercise on health status and pathophysiology.
Consequently, there is no universal protocol for resistance training or the functional assessment of strength in animal models, so versatile protocols are needed.
The aim of this study is to identify the most relevant factors to be considered when designing and applying a protocol for resistance training and evaluation using static and dynamic ladders in animal models, as well as provide specific examples.
Protocol
The methods presented in this protocol have been evaluated and approved by the animal research technical committee (reference PROAE 04/2018, Principado de Asturias, Spain).
1. Planning
- Carefully select animals for the study based on the characteristics of interest (genetically modified, pathology models, age, etc.) and apply specific adaptations to the protocol (climbing without weights, reducing the number of rungs to climb, and inclination).
- Identify the strength modality to be assessed or trained: maximal strength, endurance-resistance, speed, etc. depending on the objectives of the study.
- Adjust the parameters carefully when functional assessment or training is framed, considering whether it focuses on the results of these tests or whether they are complementary to other types of clinical, functional, histological, or molecular determinations.
- Plan all issues related to training, particularly the timetable, duration of the training period, and frequency of sessions, and draw a training table.
- Specify the warm-up steps and the inclination of the ladder, which will be the same throughout the training. Specify sets, repetitions, load (based on the results of the resistance tests done prior to the training period), and rest in between, paying attention to load increases based on the previous session.
- Modify the plan, as with human training, depending on the welfare of the animal. Modifications include decreasing repetitions, increasing rest time between sets or repetitions, and decreasing load to avoid overtraining and injury.
- Upon completion, submit the design for evaluation and approval by the animal ethics research committee.
2. Devices and materials for resistance exercise
- Devices: Static and dynamic ladders
NOTE: Two types of ladders, so-called static and dynamic ladders (see Figure 1), can be used for resistance training and evaluation (see Table of Materials).- Use a vertical ladder with at least 30 steel wire steps of 1.5 mm diameter, separated by 15 mm, and a resting area of at least 20 x 20 cm on the top of the ladder. The slope of the ladder must be adjustable from 80° to 110° with the horizontal plane (Figure 1C). Delimit two lanes to prevent non-linear climbing.
- Use a dynamic ladder similar to the static ladder, with a plastic filament barrier at the top, that can be opened to control access to the resting area, and a plastic filament barrier at the bottom, to prevent the animals from climbing down. The angle of inclination of the ladder must be adjustable between 80° and 100°, the most common being 85°.
NOTE: The ladder can circulate by means of an upper and a lower shaft with a diameter of 8 cm. Lower shaft is driven by an electric motor which makes the steps descend at the front and ascend at the rear, creating an endless ladder. It is equipped with a reduction gear and a speed regulator for lowering the speed from 11.6 cm/s to 3.3 cm/s, and the most common speed is 5.6 cm/s.
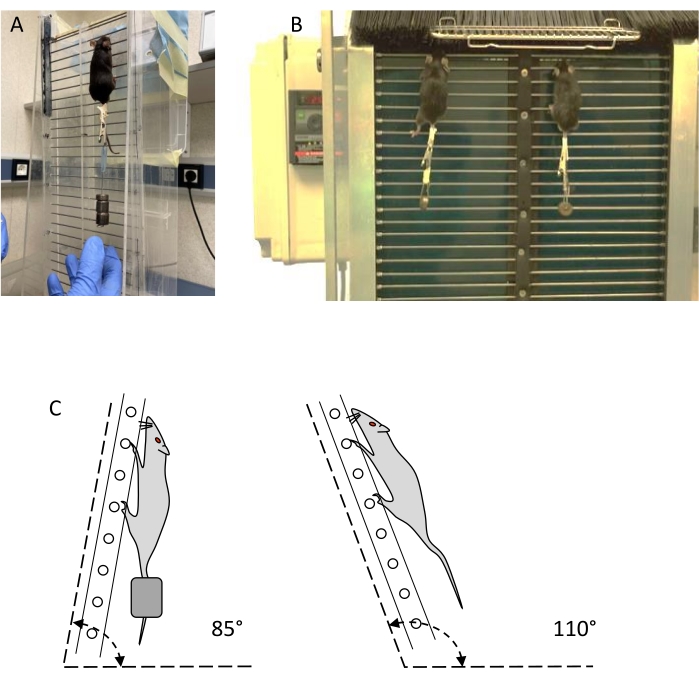
Figure 1: Resistancetraining devices: static and dynamic ladders. (A) Mouse training with external weight on a static ladder. (B) Two mice training with weight on a dynamic ladder. (C) Schematic representation of ladder angles for training and evaluation. Please click here to view a larger version of this figure.
- Materials
- Prepare the following materials: weights, wire for holding weights, steel gator clip, and clinical adhesive tape.
NOTE: The weights are steel cylinders of different mass (5, 10, 15, 20, 25, and 50 g), with a 5 mm diameter hole in the center to string them on a wire (Table of Materials). The wire to hold the weights is made of steel with a diameter of 1-1.5 mm and a length of 5-10 cm, depending on the number of weights to be loaded. - Cut a piece of elastic adhesive bandage (Table of Materials) of approximately 3.0-3.5 cm x 1.0-1.5 cm size and attach it around the animal's tail to hold the weights. Be sure not to over-tighten as it may lead to blood flow restriction.
NOTE: At first, the animals' behavior will be fighting against the tape and bitting it, but after a couple of days, they will tolerate it, grooming as usual and showing no signs of stress. - Insert the desired weights in the wire and hook the gator clip (Table of Materials: Steel gator clip and wire for holding weights).
- Clamp the gator to the clinical tape attached to the animal's tail.
- Immediately after climbing the required rungs, remove the clamp and allow the animal to rest with the clinical tape on the tail, but without the weight (Figure 1).
- Prepare the following materials: weights, wire for holding weights, steel gator clip, and clinical adhesive tape.
3. Acclimatization
NOTE: Proper acclimatization is essential to avoid exercise rejection and to minimize stress. Acclimatization is a crucial stage before resistance evaluation tests or training protocols are performed. Adequate time should be spent to achieve behavioral signs of comfort in the animals. Details of daily acclimatization with the static and dynamic ladders are shown in Table 1 and Table 2, respectively.
- Accustom the animals to stay in the resting area at the top of the ladder (static or dynamic). Leave the animals in this place in groups of four, with bedding from their cage, for 15 min every day. Usually, after 3-5 days, the animals will show no signs of stress.
- Teach animals to climb up, not down, the ladder. Using the static ladder, place the mice on a rung close to the top, from where they can see the resting area. They will instinctively go to it. Then, teach them progressively to climb up from five rungs (3x) the first day, to 10 rungs (3x) the following day, up to 15 rungs (3x) (Table 1).
Use the same procedure with the dynamic ladder, first without movement, and then with the ladder moving at 5.4 cm/s and 6.6 cm/s and the animals climbing up for 2 min, completing five series (Table 2). - Adapt the animals to carry weights, starting from the third day of acclimatization. Stick a piece of clinical tape to the base of the tail which will be used to hold weights.
- From the seventh day of acclimatization, attach small weights (5-10 g) to the clinical tape with a gator clip. Avoid performing too many series, so the adaptation is not transformed into training.
NOTE: Acclimatization of the control group is mandatory in case this group performs the resistance test. After this period, perform a ladder-climbing reminder once a week, with tape but without weights.
4. Resistance evaluation
- Incremental tests to assess maximal strength
NOTE: This test intends to determine the maximal resistance measured as the maximum weight at which the animals can climb 10 rungs on the static ladder, which defines the 10-repetition maximum (10 RM)4. This protocol was adapted from previous studies (reviewed in Kregel et al.15).- For warming-up perform three series of 10-repetitions, 10 steps/repetition, without external load. For the first series set the slope at 90°, and thereafter at 85°. Allow a rest period of 60 s between series.
- Set the slope at 85° (to prevent the weights from grazing or hooking on the rungs of the ladder).
- Attach the tape around the animal's tail to hold the weights and prepare the weights as previously explained.
- Start the test with an external load of 10 g and perform one series of 10 steps.
- Remove the weight and allow a rest period of 120 s in the resting area.
- Perform successive series of 10 steps increasing the external load by 5 g until exhaustion. Allow the resting period (120 s) between series.
- If one animal fails to climb 10 steps with a particular weight load, allow for another attempt with the same load after 120 s of rest. If it succeeds to climb with the load, it continues the test with the next load. If it fails again, record the weight load of the last completed series as its maximal weight load.
- The test result can be expressed as absolute external weight (g), as maximum load in relation to body weight (%), or as the mass lifted per gram of body weight, as per the discretion of the researcher.
NOTE: The previous protocol represents a model on which numerous modifications are possible for example, to assess the maximal resistance of genetically modified mice with neuromuscular disabilities. These animals are not able to climb with external loads and have difficulties climbing 10 rungs with the ladder set at 90° of slope (unpublished data). The protocol consisted of climbing five steps without external load, starting with a slope of 110°. The slope decreased 5° in each series until 85° with 120 s rest after each series. In this case, maximal resistance was expressed as the accumulated number of steps climbed (without considering repetitions after failures). The wild-type control group, after reaching the 85° slope, will continue with the test by adding external weight to the tail, following previous protocol, until exhaustion.
- Maximal endurance-resistance test with the static ladder
- For warming-up perform three series of 10-repetitions, 10 steps/repetition, without external load. For the first series, set the slope at 90°, and thereafter at 85°. Allow a rest period of 60 s between series.
- Set the slope at 85°.
- Clip the weight on the clinical tape placed around the tail of the mouse.
NOTE: Depending on the age and the characteristics of the animals, the external load can be the maximum weight obtained in a previous incremental test, a percentage of it (e.g., 50%), or a percentage of body weight (e.g., 100%-200%). If this test is performed after a period of training, it is recommended to use the same load as in the initial test to assess the changes. - Perform consecutive series of 10 steps until exhaustion. No resting time is allowed after each series.
- The test result is the number of climbed rungs.
- Maximal endurance-resistance test with the dynamic ladder
NOTE: The use of the dynamic ladder allows the researcher to control the climbing speed.- Set the slope at 85°.
- Set the speed at 4.2 cm/s.
- For warming-up perform three series of 100 steps, without external load. Allow a rest period of 60 s between series.
- Clip the weight on the clinical tape placed around the mouse tail.
NOTE: Depending on the age and the characteristics of the animals, the external load can be the maximum weight obtained in a previous incremental test, a percentage of it (e.g., 50%), or a percentage of body weight (e.g., 100%-200%). If this test is performed after a period of training, it is recommended to use the same load as in the initial test to assess the changes. - Start at 4.2 cm/s and increase the speed by 1.2 cm/s every 60 s until exhaustion.
NOTE: The test result is the exercise time, the number of rungs climbed, or the maximum speed.
5. Resistance training with static ladder
NOTE: Before starting the training period, acclimatization (Table 1) and training planning are necessary. To reduce anxiety, adapt and train the mice in groups of four animals sharing the same cage.
- For everyday warming-up perform three series of 10 repetitions, 10 steps/repetition, without external load. For the first series set the slope at 90°, and thereafter at 85°. Allow a rest period of 60 s between series.
- The training session starts in the resting area. Clip the gator with the weight on the clinical tape.
- Gently place the mouse 10-20 rungs below the resting place. Allow the mouse to grip the rung and climb to the resting area.
Repeat this process until the number of rungs in this series (e.g.,10 rungs x 10 series) is completed. - Remove the weight from the mouse tail and wait for 120 s until the next series.
- Increase the number of steps and the maximum weight loads of the series throughout the training period, while maintaining the weekly schedule.
NOTE: An example of the variation of loads during a week planning is shown in Table 3. Shortly, Tuesday and Friday with high weight load (40-50 g) and a low number of steps (500-400); Monday and Thursday with intermediate weight load (25-35 g) and an intermediate number of steps (800-600); and Wednesday without weight load but a high number of steps (2,000). This design facilitates recovery from previous training sessions and avoids injuries and overtraining. Examples of 3 weeks of training with multiple designs using the static ladder are shown in Table 4 (at the beginning, in the middle, and at the end of the training period, respectively)4.
6. Resistance training with dynamic ladder
NOTE: After acclimatization, the training on the dynamic ladder is quite like the static one (Table 2). Training is performed on 2-4 mice at a time.
- Set the slope to 85°, close the door to the resting area, and start the ladder at the desired speed (e.g., 5.4 cm/s).
- For warming-up perform three series of 100 steps, without external load. Allow a rest period of 60 s between series.
- Before the training sessions starts, when the mouse is in the resting area, clip the gator with the weight on the clinical tape. Alternatively, the weight can be attached when the mouse is already on the ladder.
- Gently place the mouse at the top of the moving staircase with the weight on the tail. Allow the mice to grip the rung and climb.
- When the number of rungs in this series is reached (e.g., 100), remove the weights. Then the door is opened so that the animal can go to the resting area. The rest time is 120 s before the next series.
NOTE: The number of steps climbed is counted as a function of the climbing time at the set speed. - Repeat this procedure until the training session is completed. The detailed daily training program is shown in Table 5.
7. Evaluation of the crossover effect of resistance training on endurance performance
NOTE: For this, an incremental treadmill test is performed4, after 24 h of rest.
- After a warm-up of 3 min at 10 cm/s, start the incremental test at 10 cm/s and 10° angle of inclination.
- Increase the speed by 3.33 cm/s every 3 min until exhaustion.
NOTE: No electric shocks are used, so a painter's brush is placed at the back of the treadmill to prevent the mice from running off it.
8. Animal behavior during procedures
NOTE: Continuous monitoring of the adaptation of mice to training should be performed to detect extreme fatigue, overtraining, or injury.
- Observe signs of animal welfare, in particular grooming and refusal to training. The normal behavior of the mouse, after a series of intense training, is to remain inactive for about one minute due to fatigue. After that, they start grooming, exploring, or trying to remove the tape on the tail.
- In the case of a mouse refusing to train a series, try giving longer rests or even not performing that series to prevent inhibition.
- Occasionally, when carrying out lightweight exercises, gently push the animal's tail, to encourage it to finish the series. The animals stop climbing because it is not a demanding task. Conversely, when animals are carrying a heavy load, gently shift the animal's weight to ease the load and encourage it to finish the series, and then allow the animal to rest until the next training session. The animals may stop or even attempt to descend because of the heavy load.
9. Safety procedures
- Security procedures for researchers: Conduct research in the animal facility laboratory and use shoe covers, coveralls, gloves, caps, and masks. There are no additional requirements other than those specific to animal research.
- Security for animals: During the exercise sessions continuous attention must be paid to the animals, due to potential risks, such as falls or jumps. Place a hand under the weights to catch and hold the mice in case of fall due to exhaustion, since its ability to hold on to the rungs properly will be limited.
Results
Results with static ladder
The progressive resistance training protocol used and described by Codina-Martinez et al.4 (Table 4) was tested in a preliminary study consisting of 7 weeks of training on a static ladder with 6-months-old wild-type C57BL6J mice (n = 4). In this preliminary study, incremental tests to assess maximal strength were performed before and after the training period. We observed a 46.4% increase in maximal strength, meaning that at the en...
Discussion
Training is an intervention with multiple applications in research, apart from the study of exercise itself. Thus, the analysis of its effect on ageing20 or certain pathological conditions and physical therapy21 has received much attention in recent years. In addition, numerous authors have analyzed the effect of pharmacological22 or dietary21 interventions on physical fitness. In this context, interest has...
Disclosures
The corresponding author ensures that all authors have no conflicts of interest.
Acknowledgements
This work was supported in part by the Ministerio de Economía y Competitividad, Spain (DEP2012-39262 to EI-G and DEP2015-69980-P to BF-G). Thanks to Frank Mcleod Henderson Higgins from McLeod´s English Centre in Asturias, Spain, for language assistance.
Materials
| Name | Company | Catalog Number | Comments |
| Dynamic ladder | in-house production | ||
| Elastic adhesive bandage 6 cm x 2.5 m | BSN medical | 4005556 | |
| Gator Clip Steel NON-INSUL 10A | Digikey electronics | BC60ANP | |
| Static ladder | in-house production | ||
| Weights | in-house production | ||
| Wire for holding weigths | in-house production |
References
- Pedersen, B. K., Saltin, B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scandinavian Journal of Medicine & Science in Sports. 25, 1-72 (2015).
- Westcott, W. L. Resistance training is medicine: effects of strength training on health. Current Sports Medicine Reports. 11 (4), 209-216 (2012).
- Garatachea, N., et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Research. 18 (1), 57-89 (2015).
- Codina-Martinez, H., et al. Autophagy is required for performance adaptive response to resistance training and exercise-induced adult neurogenesis. Scandinavian Journal of Medicine & Science in Sports. 30 (2), 238-253 (2020).
- Conner, J. D., Wolden-Hanson, T., Quinn, L. S. Assessment of murine exercise endurance without the use of a shock grid: an alternative to forced exercise. Journal of Visualized Experiments: JoVE. (90), e51846 (2014).
- Meijer, J. H., Robbers, Y. Wheel running in the wild. Proceedings of the Royal Society B: Biological Sciences. 281 (1786), 20140210 (2014).
- Suchomel, T. J., Nimphius, S., Bellon, C. R., Hornsby, W. G., Stone, M. H. Training for muscular strength: Methods for monitoring and adjusting training intensity. Sports Medicine. 51 (10), 2051-2066 (2021).
- Pousson, M., Perot, C., Goubel, F. Stiffness changes and fibre type transitions in rat soleus muscle produced by jumping training. Pflügers Archive. 419 (2), 127-130 (1991).
- Marqueti, R. C., et al. Biomechanical responses of different rat tendons to nandrolone decanoate and load exercise. Scandinavian Journal of Medicine & Science in Sports. 21 (6), 91-99 (2011).
- Cunha, T. S., Tanno, A. P., Costa Sampaio Moura, M. J., Marcondes, F. K. Influence of high-intensity exercise training and anabolic androgenic steroid treatment on rat tissue glycogen content. Life Sciences. 77 (9), 1030-1043 (2005).
- Heinemeier, K. M., et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. The Journal of Physiology. 582, 1303-1316 (2007).
- Hornberger, T. A., Farrar, R. P. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Canadian Journal of Applied Physiology. 29 (1), 16-31 (2004).
- Yarasheski, K. E., Lemon, P. W., Gilloteaux, J. Effect of heavy-resistance exercise training on muscle fiber composition in young rats. Journal of Applied Physiology. 69 (2), 434-437 (1990).
- Khamoui, A. V., et al. Aerobic and resistance training dependent skeletal muscle plasticity in the colon-26 murine model of cancer cachexia. Metabolism. 65 (5), 685-698 (2016).
- Kregel, K. C., et al. Resource book for the design of animal exercise protocols. American Physiological Society. 152, (2006).
- Marino, G., et al. Autophagy is essential for mouse sense of balance. The Journal of Clinical Investigation. 120 (7), 2331-2344 (2010).
- Figueiredo, V. C., de Salles, B. F., Trajano, G. S. Volume for muscle hypertrophy and health outcomes: The most effective variable in resistance training. Sports Medicine. 48 (3), 499-505 (2018).
- Gentil, P., et al. Using velocity loss for monitoring resistance training effort in a real-world setting. Applied Physiology, Nutrition, and Metabolism. 43 (8), 833-837 (2018).
- Fernández-Sanjurjo, M., et al. Is physical performance (in mice) increased by Veillonella atypica or decreased by Lactobacillus bulgaricus. Journal of Sport and Health Science. 9 (3), 197-200 (2020).
- Shiguemoto, G. E., et al. Effects of resistance training on matrix metalloproteinase-2 activity and biomechanics and physical properties of bone in ovariectomized and intact rats. Scandivavian Journal of Medicine & Science in Sports. 22 (5), 607-617 (2012).
- de Sousa Neto, I. V., et al. Effects of resistance training on matrix metalloproteinase activity in skeletal muscles and blood circulation during aging. Frontiers in Physiology. 9, 190 (2018).
- Ghosh, S., Golbidi, S., Werner, I., Verchere, B. C., Laher, I. Selecting exercise regimens and strains to modify obesity and diabetes in rodents: an overview. Clinical Science. 119 (2), 57-74 (2010).
- Mônico-Neto, M., et al. Resistance training minimizes catabolic effects induced by sleep deprivation in rats. Applied Physiology, Nutrition, and Metabolism. 40 (11), 1143-1150 (2015).
- Hawley, J. A., Hargreaves, M., Joyner, M. J., Zierath, J. R. Integrative biology of exercise. Cell. 159 (4), 738-749 (2014).
- Booth, F. W., Laye, M. J., Spangenburg, E. E. Gold standards for scientists who are conducting animal-based exercise studies. Journal of Applied Physiology. 108 (1), 219-221 (1985).
- Kruger, K., et al. Functional and muscular adaptations in an experimental model for isometric strength training in mice. PLoS One. 8 (11), 79069 (2013).
- Hendrickse, P. W., Krusnauskas, R., Hodson-Tole, E., Venckunas, T., Degens, H. Endurance exercise plus overload induces fatigue resistance and similar hypertrophy in mice irrespective of muscle mass. Experimental Physiology. 105 (12), 2110-2122 (2020).
- Knab, A. M., et al. Repeatability of exercise behaviors in mice. Physiology & Behavior. 98 (4), 433-440 (2009).
- Konhilas, J. P., et al. Loaded wheel running and muscle adaptation in the mouse. American Journal of Physiology-Heart and Circulatory Physiology. 289 (1), 455-465 (2005).
- Reiter, A., et al. Functional measures of grip strength and gait remain altered long-term in a rat model of post-traumatic elbow contracture. The Journal of Biomechanical Engineering. , (2019).
- Stieglitz, T., Schuettler, M., Schneider, A., Valderrama, E., Navarro, X. Noninvasive measurement of torque development in the rat foot: measurement setup and results from stimulation of the sciatic nerve with polyimide-based cuff electrodes. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 11 (4), 427-437 (2003).
- Seo, D. Y., et al. Humanized animal exercise model for clinical implication. Pflügers Archiv. 466 (9), 1673-1687 (2014).
- Tanaka, H., Swensen, T. Impact of resistance training on endurance performance. A new form of cross-training. Sports Medicine. 25 (3), 191-200 (1998).
- Hakkinen, K., Mero, A., Kauhanen, H. Specificity of endurance, sprint and strength training on physical performance capacity in young athletes. The Journal of Sports Medicine and Physical Fitness. 29 (1), 27-35 (1989).
- Vellers, H. L., Kleeberger, S. R., Lightfoot, J. T. Inter-individual variation in adaptations to endurance and resistance exercise training: genetic approaches towards understanding a complex phenotype. Mammalian Genome. 29 (1), 48-62 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved