A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Methodology to Test Control Agents and Insecticides Against the Coffee Berry Borer Hypothenemus hampei
In This Article
Summary
A method using green coffee fruits (GFs) was developed to test the toxicity of insecticides against the coffee berry borer (CBB). Insecticides or toxic substances were applied to disinfected GFs before or after CBB infestation. Insect mortality, repellency, and reproductive capacity, in addition to other parameters, were evaluated.
Abstract
Prior to recommending insecticides to treat the coffee berry borer (CBB) Hypothenemus hampei, it is valuable to know the mortality and repellency of these insecticides against adult insects or their impact on reproductive output. However, currently available methods assess adult mortality only, limiting the selection of novel insecticides with a different mode of action. In this work, different experimental methods were examined to identify the diverse effects on the CBB under laboratory conditions. For this, green coffee fruits (GFs) were collected and disinfected by immersion in sodium hypochlorite solution followed by UV light irradiation. In parallel, CBB adults from a colony were disinfected by immersion in sodium hypochlorite solution. To assess fruit protection (preinfestation), the fruits were placed in plastic boxes, and the insecticides were applied. Then, the CBB adults were released at a rate of two CBBs per GF. The GFs were left under controlled conditions to evaluate CBB infestation and survival after 1, 7, 15, and 21 days. To evaluate insecticide efficacy after CBB infestation (postinfestation), CBB adults were released to the GFs in a 2:1 ratio for 3 h at 21 °C. Infested fruits showing CBB adults with their abdomens partially exposed were selected and placed in 96-well racks, and the CBBs boring into the fruits were treated directly. After 20 days, the fruits were dissected, and the CBB biological stages inside each fruit were recorded. The GFs served as substrates that mimic natural conditions to evaluate toxic, chemical, and biological insecticides against the CBB.
Introduction
The coffee berry borer (CBB), Hypothenemus hampei, was first detected in 1988 in Colombia and has since become the most important pest species of the coffee crop. CBB females leave the natal fruit already fertilized, seeking new fruits guided by the volatile chemicals that they emit1,2. A complete cycle is fulfilled within 23 days3 at a temperature of 25 °C. The cycle starts with the founder female penetrating the seed and laying eggs in the fruit endosperm. The eclosed larvae eat the seed. If the fruits are dissected at this point, it would be possible to observe both the founder female and her offspring. After 14 days, the larvae become pupae-generally, the pupae stage lasts 5 days. In the adult stage, the females copulate with their siblings, and the newly fertilized females fly away from the damaged fruits looking for new coffee fruits to start a new cycle4.
Both the penetration process and the result of larval feeding damage the coffee seed, decreasing the quality of the coffee beverage and significantly reducing the revenue; greater than 5% infestation in coffee plantations is generally considered the economic threshold.
CBB control is based on an integrated pest management (IPM) strategy, including cultural control and agronomic practices, natural biological agents, and the use of chemical insecticides, which requires safety conditions and timely application4.
To evaluate new insecticides for the control of the CBB, low-cost methodologies are needed that allow rapid results to be obtained. Both laboratory and field procedures are currently in use, including artificial diets containing coffee in which the insecticides are incorporated5,6, or spraying the insecticides on dry parchment coffee7,8,9. In addition, experiments carried out in the field using coffee tree branches covered with entomological sleeves have been reported10,11; however, these methods require intense labor and long evaluation periods.
A condition resembling natural field conditions, that is also fast and inexpensive, is the use of green or ripe coffee fruits. However, these fruits must be maintained under conditions suitable for developing the CBB, avoiding alterations and contaminants by microorganisms to maintain their quality and properties. To this end, different disinfectants have been used, as well as procedures involving heat and radiation7,9,12,13,14,15,16.
Additionally, the methods for insecticide evaluation against the CBB require simulations of adult females flying in search of fruits or penetrating those fruits17,18. For this, artificial fruit infestations have been carried out in the field8,11,19, although this process is labor-intensive and depends on environmental conditions.
Here, we describe a standardized methodology for the evaluation of products that can have different effects on the CBB under controlled environmental conditions that resemble field conditions.
Access restricted. Please log in or start a trial to view this content.
Protocol
NOTE: This protocol addresses different methods to identify different effects on the CBB under laboratory conditions.
1. Fruit collection
- Pick GFs with a developmental age of ~120-150 days after flowering from trees in a coffee plantation early in the morning.
2. Fruit disinfection20
- Bring around 300 GFs to the laboratory. Select uniformly sized and healthy GFs and withdraw the peduncles.
- Dip the GFs into a soap solution (2 mL of liquid dishwashing soap in 998 mL of tap water), followed by rubbing to wash the GFs. Then, rinse the fruits with water, changing the water three times.
- Immerse GFs in 0.5% sodium hypochlorite solution (100 mL in 900 mL of tap water) and stir in a shaker at 110 rpm for 15 min. Then, rinse the GFs with water by stirring in a shaker and changing the water three times, every 10 min.
- Dry the GFs with sterile paper towels.
- Place the GFs in trays (33 cm x 25 cm x 2 cm) and irradiate them for 15 min, placing the GFs at a distance of 55 cm from the UV source inside a UV-enabled horizontal laminar flow station.
- During the 15 min period, every 5 min, move the GFs to ensure irradiation of the whole fruit.
3. Insect disinfection21
- Use newly emerged (same-day) CBB insects to set up the bioassays.
- Immerse the CBBs in 0.5% sodium hypochlorite solution, agitating them slowly with a brush for 10 min.
- Filter the CBBs through a muslin cloth and wash them three times with sterile distilled water.
- Remove excess water with sterile paper towels.
4. Evaluation of a product with a protective effect on the fruits (preinfestation) (Figure 1)
- Use a group of GFs per experimental unit. Generally, a group of 30 GFs are used per experimental unit.
- Place the GFs in plastic boxes (experimental unit).
- Apply the test product at the different concentrations for evaluation. Carry out the application with a portable sprayer unit. Here an alkaloid emulsion at 5% and 6% were tested.
- As a control, spray a group of GFs with water.
- Utilize at least three repetitions (experimental unit) per treatment, spraying one after the other.
- In a sterile hood, release two CBB adults per GFs (a total of 60 CBBs are introduced into the plastic boxes). After 30 min, cover the boxes.
- Leave the plastic boxes with the infested GFs in a room or incubator under controlled conditions (dark, 25 ± 2 °C, and relative humidity 71% ± 5%).
- After 1, 7, 15, and 21 days, count the number of borer fruits and living and dead insects outside the fruits in each box.
- At 20 days postinfestation, dissect each GF under a stereomicroscope, magnification 10x.
- Count the number of healthy seeds or seeds damaged by the insects in each fruit.
- Count the different CBB biological stages22 observed and count the number of dead insects in each seed to determine insect mortality per experimental unit.
5. Evaluation of the effect of a product after CBB infestation (postinfestation) (Figure 3)
- Use groups of 200 fruits per treatment.
- In asterile hood,release CBB adults (2:1 ratio of CBB adults to GFs) to the previously disinfected GFs, allowing the infestation to proceed for 3 h at 21 °C.
- Examine the GFs. After 3 h, most should be infested, with the abdomen of the CBBs still exposed (position A20), as shown in Figure 2.
- Select 46 infested GFs (position A) and place them in 96-well plastic racks (experimental unit). The fruits should remain in this position so that the treatment can be directly sprayed on the CBB perforating the fruit.
- Spray at least three time (three racks) per treatment, one after the other, covering the racks after 30 min.
- Leave the racks with the infested GFs in a room or incubator under controlled conditions (dark, 25 ± 2 °C, and relative humidity 71% ± 5%).
- After 20 days, dissect the GFs under a stereomicroscope at 10x magnification.
- Count the number of healthy seeds or seeds damaged by the insects in each fruit.
- Count the different CBB biological stages22 and the number of dead insects in each seed to determine insect mortality per experimental unit.
6. Evaluation of a product with a deterrent effect on the CBB
- Follow the steps 4.1-4.6 outlined for evaluating a product with a protective effect on the fruits.
- After releasing the CBB adults into the plastic boxes, count the number of CBBs that fly away from the boxes and the number that infest the GFs. Then, follow steps 4.7-4.11.
- Follow the steps 5.1-5.5 outlined for evaluating the product after the CBB infestation.
- After spraying each treatment on the insects in position A, count the number of CBBs that moved out of the GF and/or flew away from the GF. Then, follow steps 5.6-5.9.
7. Statistical analysis
NOTE: The response variables are mortality percentages over time and the percentage of healthy uninfested coffee seeds.
- Estimate the average and standard deviation of each response variable for each treatment.
- Perform analysis of variance for each response variable with a model for a completely randomized design.
NOTE: Dunnett's 5% comparison test is performed to compare the treatments against the absolute control (water control). - When the treatments are significantly different from the absolute control, use a 5% least significant difference (LSD) test to compare the treatments.
- Evaluate the power of the test; if greater than 85%, the assumptions of normality and homogeneity of the variances are met.

Figure 1: Procedure for the evaluation of the preinfestation effects of insecticides on the CBB. Steps for evaluating the preinfestation effects of insecticides on Hypothenemus hampei (CBB) using green fruits (GFs). (A) Fruit selection. (B) Spraying of the insecticides on the coffee fruits. (C) CBB infestation of coffee fruits at a ratio of 2:1 CBB per GF. (D) Infested fruits. (E) Incubation of the fruits under controlled conditions. (F) Fruit dissection. (G) Counting the CBB population inside the seeds. Please click here to view a larger version of this figure.

Figure 2: Process coffee fruits' CBB infestation. The infested fruits contain CBB adults with their abdomens partially exposed (position A). Please click here to view a larger version of this figure.
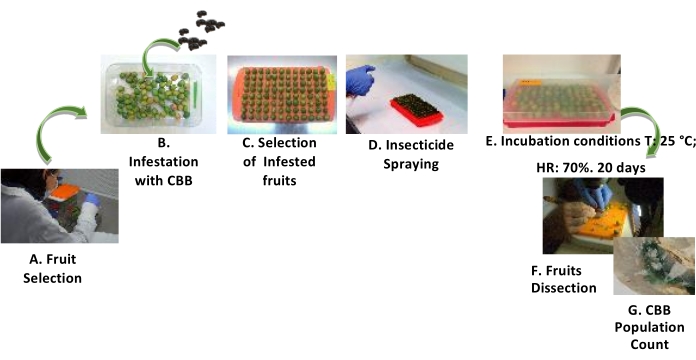
Figure 3: Procedure for the evaluation of the posinfestation effects of insecticides on the CBB. Steps for evaluating the postinfestation effects of insecticides on the CBB using GFs. (A) Fruit selection. (B) Infestation of the fruits with CBB at a ratio of 2:1 CBB per GF. (C) Selection of infested fruits. (D) Spraying of the insecticide on the fruits. (E) Incubation of the fruits. (F) Fruit dissection. (G) Counting the CBB population. Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Results
The results showed that the CBB females recognized the fruits, and depending on the characteristics of the fruit surface and the emitted odors, the CBB females started to penetrate or bore the fruits within 3 h at 21 °C.
The effect of an insecticide on the CBB when applied to the coffee fruits (preinfestation procedure) after 24 h and over time is shown in Figure 4. The two insecticides (alkaloid emulsion at 5% and 6%) caused high insect mortality on Day...
Access restricted. Please log in or start a trial to view this content.
Discussion
In this protocol, disinfection of the fruits as well as the insects are critical steps. When fruits from the field are used in the laboratory, they frequently show high contamination and dehydration since microorganisms and mites are present in the epidermis7,15,16. Therefore, using fruits or insects that are not disinfected will cause insect death due to contamination caused by microorganisms, such as bacteria or fungi, thus in...
Access restricted. Please log in or start a trial to view this content.
Disclosures
None of the authors have any conflicts of interest to declare.
Acknowledgements
The authors express their thanks to the National Federation of Coffee Growers of Colombia, the assistants of the Department of Entomology (Diana Marcela Giraldo, Gloria Patricia Naranjo), Experiment Station Naranjal, and Jhon Félix Trejos.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Beaker with spout, low form 500 mL | BRAND PP | BR87826 | |
| Benchtop Shaker | New Brunswick Scientific Innova 4000 Incubator Shaker | ||
| Dishwashing liquid soap-AXION | Colgate-Palmolive | AXION | |
| Hood; Horizontal Laminar Flow Station | Terra Universal | Powder-Coated Steel, 1930 mm W x 1118 mm D x 1619 mm H, 120 V (https://www.terrauniversal.com/hood-horizontal-laminar-flow-station-9620-64a.html) | |
| Insects CBB | BIOCAFE | (http://avispitas.blogspot.com/p/biocafe.html). | |
| Multi Fold White paper towels | Familia | 73551 | |
| Preval Spray unit | Preval Merck | Z365556-1KT | https://www.sigmaaldrich.com/CO/es/product/sigma/z365556?gclid=Cj0KCQiAweaNBhDEARIsAJ 5hwbfZOy1TWGj6huatFtRQt AzOyHe5-oBiKnOUK2T1exuuk WwJLdvxkvsaAjoYEALw_wcB |
| Reversible Racks 96-Well | heathrowscientific | HEA2345A | https://www.heathrowscientific.com/reversible-racks-96-well-i-hea2345a |
| Scalpel blades N 11 | Merck | S2771-100EA | |
| Scalpel handles N3 | Merck | S2896-1EA | |
| Sodium Hypochloride | The clorox company | Clorox | |
| Stereo Microscope | Zeiss | Stemi 508 | https://www.zeiss.com/microscopy/int/products/stereo-zoom-microscopes/stemi-508.html |
References
- Mendesil, E., et al. Semiochemicals used in host location by the coffee berry borer, Hypothenemus hampei. Journal of Chemical Ecology. 35 (8), 944-950 (2009).
- Jaramillo, J., et al. Coffee berry borer joins bark beetles in coffee klatch. PLoS ONE. 8 (9), 74277(2013).
- Giraldo-Jaramillo, M., Garcia, A. G., Parra, J. R. Biology, thermal requirements, and estimation of the number of generations of Hypothenemus hampei (Ferrari, 1867) (Coleoptera: Curculionidae) in the state of São Paulo, Brazil. Journal of Economic Entomology. 111 (5), 2192-2200 (2018).
- Benavides, P., Góngora, C., Bustillo, A. IPM Program to Control Coffee Berry Borer Hypothenemus hampei, with Emphasis on Highly Pathogenic Mixed Strains of Beauveria bassiana, to Overcome Insecticide Resistance in Colombia. IntechOpen. , (2012).
- Martínez, C. P., Echeverri, C., Florez, J. C., Gaitan, A. L., Góngora, C. E. In vitro production of two chitinolytic proteins with an inhibiting effect on the insect coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae) and the fungus Hemileia vastatrix the most limiting pests of coffee crops. AMB Express. 2, 1-11 (2012).
- Padilla, B. E., Acuña, Z., Velásquez, C. S., Rubio, G. J. D. Inhibitors of [alpha]-amylases from the coffee berry borer Hypothenemus hampei in different plant species. Revista Colombiana de Entomología. 32 (2), 125-130 (2006).
- Alvarez, J. H., Cortina, H. A., Villegas, J. F. Methods to evaluate antibiosis to Hypothenemus hampei Ferrari in coffee under controlled conditions. Cenicafé. 52 (3), 205-214 (2001).
- Arcila, A., Duarte, A. F., Villalba, D. A., Benavides, P. New Product in the Integrated Management of the Coffee Berry Borer in Colombia. National Coffee Research Center (Cenicafé). , Available from: https://biblioteca.cenicafe.org/handle/10778/477 (2014).
- Jaramillo, J., Montoya, E., Benavides, P., Góngora, C. Beauveria bassiana and Metarhizium anisopliae for the control of coffee brocade in fruits on the ground. Revista Colombiana de Entomología. 41, 95-104 (2015).
- Bastidas, A., Velásquez, E., Benavides, P., Bustillo, A., Orozco, C. Evaluation of preformulated Beauveria bassiana (Bálsam) Vuillemin, for the control of the coffee berry borer. Agronomia. 17, 44-61 (2009).
- Villalba-Gault, D., Bustillo, A., Chaves Cordoba, B. Evaluation of insecticides for the control of the coffee berry borer in Colombia. Cenicafe. 46, 152-163 (1995).
- Bustillo, A. E., Orozco, J., Benavides, P., Portilla, M. Mass production and use of parasitoids for the control of the coffee berry borer in Colombia. Cenicafe. 47 (4), 215-230 (1996).
- Celestino, F. N., Pratissoli, D., Machado, L. C., Santos Junior, H. J. G. D., Mardgan, L., Ribeiro, L. V. Adaptation of breeding techniques of the coffee berry borer [Hypothenemus hampei (Ferrari). Coffee Science. 11 (2), 161-168 (2016).
- Domínguez, L., Parzanese, M. Ultraviolet light in food preservation. Argentine Foods. 52 (2), 70-76 (2012).
- Jaramillo, J., Chabi-Olaye, A., Poehling, H. M., Kamonjo, C., Borgemeister, C. Development of an improved laboratory production technique for the coffee berry borer Hypothenemus hampei, using fresh coffee berries. Entomologia Experimentalis et Applicata. 130 (3), 275-281 (2009).
- Pérez, J., Infante, F., Vega, F. E. Does the coffee berry borer (Coleoptera: Scolytidae) have mutualistic fungi. Annals of the Entomological Society of America. 98 (4), 483-490 (2005).
- Benavides, P., Gil, P., Góngora, C., Arcila, A. Integrated pest management. Cenicafe. Manual of the Colombian coffee grower: Research and technology for the sustainability of coffee growing. Manizales: FNC: Cenicafé. 3, 179-214 (2013).
- Bustillo, P. A review of the coffee berry borer, Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae), in Colombia. Revista Colombiana de Entomología. 32 (2), 101-116 (2006).
- Arcila, A., Benavides, P., Mejia, J. New Chemical Control Alternative for the Integrated Management of the Coffee Berry Borer. National Coffee Research Center (Cenicafé). , Available from: https://biblioteca.cenicafe.org/handle/10778/557 (2015).
- Tapias, L., Martinez, C., Benavides, P., Gongora, C. Laboratory method to evaluate the effect of insecticides on the coffee berry borer. Cenicafé. 68 (2), 76-89 (2017).
- Bustillo, A. E., Marín, P. How to reactivate the virulence of Beauveria bassiana to control the coffee berry borer. Manejo Integrado de Plagas. 63, (2002).
- Constantino, L. M., et al. morphological and genetic aspects of Hypothenemus obscurus and Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae). Revista Colombiana de Entomología. 37 (2), 173-182 (2011).
- Estrela, C., et al. Mechanism of action of sodium hypochlorite. Brazilian Dental Journal. 13 (2), 113-117 (2002).
- Diffey, B. L. Solar ultraviolet radiation effects on biological systems. Physics in Medicine and Biology. 36 (3), 299-328 (1991).
- BIOCAFE. , Available from: http://avispitas.blogspot.com/p/biocafe.html (2022).
- Bustillo, A. E., et al. Integrated Management of the Coffee Berry Borer: Hypothenemus hampei Ferrari in Colombia. , Available from: https://biblioteca.cenicafe.org/hangle/10778/848 (1998).
- Portilla, R. Development and evaluation of an artificial diet for the rearing of Hypothenemus hampei. Cenicafé. 50, 24-38 (1999).
- Portilla, R. M., Streett, D. A. New techniques for automated mass production of Hypothenemus hampei on the modified Cenibroca artificial diet. Cenicafé. 57, 37-50 (2006).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved