A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Mice Model of Chlorhexidine Gluconate-Induced Peritoneal Damage
* These authors contributed equally
In This Article
Summary
The present protocol establishes a peritoneal dialysis (PD) mouse model of chlorhexidine gluconate (CG)-induced peritoneal fibrosis. The current model is simple and easy to use compared to other PD animal models.
Abstract
Peritoneal fibrosis is an important complication of peritoneal dialysis (PD). To investigate and address this problem, an appropriate animal model of PD is required. The present protocol establishes a chlorhexidine gluconate (CG) induced peritoneal fibrosis model that mimics the condition of a patient with PD. Peritoneal fibrosis was induced by intraperitoneal injection of 0.1% of CG in 15% ethanol for 3 weeks (administered every other day), for a total of nine times in male C57BL/6 mice. Peritoneal functional tests were then performed on day 22. After the mice were sacrificed, the parietal peritoneum of the abdominal wall and the visceral peritoneum of the liver were harvested. They were thicker and more fibrotic when analyzed microscopically after Masson's trichrome staining. The ultrafiltration rate decreased, and glucose mass transport indicated a CG-induced increase in peritoneal permeability. The PD model thus established may have applications in improving PD technology, dialysis efficacy, and prolonging patient survival.
Introduction
Peritoneal dialysis (PD) is a type of renal replacement therapy. However, PD has problems that cannot be solved. For example, long-term PD treatment can cause peritoneal damage, eventually leading to ultrafiltration failure and withdrawal of treatment1,2,3,4,5,6. Peritoneal fibrosis is one of the most serious complications7,8. Peritoneal fibrosis is characterized by the deposition and accumulation of extracellular matrix within the interstitium, and neo-angiogenesis and vasculopathy of the peritoneum9,10.
The main causes of these peritoneal changes are recurrent peritonitis and non-biocompatibility of the dialysate, which are hyperosmotic, high glucose, low pH, and glucose degradation product accumulation11,12. Therefore, suitable animal experimental models can help researchers better study the peritoneum's physiological and pathological changes during PD therapy. Therefore, establishing an animal PD model is important for improving PD technology and dialysis efficacy and prolonging patient survival. This study aimed to generate a PD mouse model by intraperitoneal (i.p.) injection of chlorhexidine gluconate (CG), as described previously13,14. This PD mouse model is simple, easy to use, and feasible compared to other PD animal models.
Access restricted. Please log in or start a trial to view this content.
Protocol
All mouse experiments were approved by the Laboratory Animal Center of the E-DA Hospital/ I-Shou University and were handled according to the "Guide for the Care and Use of Laboratory Animals" (NRC, USA 2011). Male C57BL/6 mice, 7-8 weeks old, were used for the present study.
1. Chemical preparation
- Prepare the chemical irritant by diluting 0.1% chlorhexidine gluconate (CG, see Table of Materials) in 15% ethanol.
2. Animal treatment
- Assign three mice as the control group. Perform intraperitoneal injection (i.p.) of 1 mL/kg of 0.9% normal saline (NS) every other day for 3 weeks, for a total of nine times.
- Assign three mice to the peritoneal fibrosis group. Induce peritoneal fibrosis using chlorhexidine gluconate (CG) by administering i.p. injections of 0.1% of CG in 15% ethanol (step 1.1) at a dose of 12.5 µL/g body weight. Perform this every other day for 3 weeks, for a total of nine times.
3. Peritoneal function tests (modified peritoneal equilibration test)
- Prepare a dialysis solution containing 4.25% glucose. Draw 0.5 mL of dialysate sample with a syringe, and then check the glucose concentration in the dialysate sample.
NOTE: The glucose concentration is determined according to the hexokinase/G6PD method. Dialysate samples were accessed to L-type Glu 2 assay and investigated with a biochemical analyzer (see Table of Materials). This is the initial dialysate glucose concentration. - Anesthetize the mice by intramuscular injection of Zoletil and Xylazine (prepared in a ratio of 1:2 by volume, see Table of Materials) at a dose of 20 µL/20 gw. Additionally, use vet ointment on the eyes to prevent dryness under anesthesia.
- Perform i.p. instillation of the dialysis solution (2 mL/20 g bodyweight).
- After 30 min, assess and verify the depth of anesthesia with lack of toe pinch reflex. Then, perform a vertical incision in the midline of the abdominal (beneath the xiphoid process), then open the mice's abdomen and collect the intraperitoneal fluid with a syringe (defined as "volume 1"). Then, measure the weight of a clean and dry cotton and put the cotton into the mice's abdominal cavity to absorb the residual intraperitoneal fluid. Finally, measure the cotton weight again.
NOTE: The weight gain of cotton is equal to the weight of the residual intraperitoneal fluid. Then, convert to the obtained volume (specific gravity: 1 g/cm3; defined as "volume 2"). The final dialysate volume is volume 1 plus volume 2. - Use 0.5 mL of dialysate sample (final dialysate) to measure the glucose concentration. This is the final dialysate glucose concentration.
- Calculate the net ultrafiltration using the formula15:
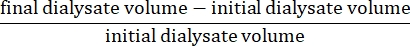
- Calculate the peritoneal permeability using the following formula15:
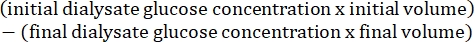
4. Tissue preparation of the abdominal wall muscle and liver and histological analysis
- Sacrifice the mice via cardiac puncture (phlebotomy)3,16.
- Cut the abdominal wall (1 cm x 1 cm) and total hepatectomy. Fix the mice's abdominal wall and liver tissues overnight in 10% neutral buffered formalin.
- Prepare 3 µm thick paraffin sections of the abdominal wall muscle and liver, and perform histological analysis following previously published report17.
- Evaluate the parietal peritoneum of the abdominal wall and visceral peritoneum of the liver surfaces of the mice using morphometry18.
- Perform statistical analyses using statistics and graphing software (see Table of Materials). Express all data as mean ± SD and analyze for statistical significance using a t-test19. Define values with P < 0.05 as significant results.
Access restricted. Please log in or start a trial to view this content.
Results
In Figure 1A,B, the parietal peritoneum of the abdominal wall was markedly thicker and more fibrotic under Masson's trichrome staining17, indicating that in the CG-exposed group, peritoneal fibrosis is more severe than in the control saline group (NS). In Figure 2A,B, the visceral peritoneum of the liver surfaces was also markedly thicker and more fibrotic, thus proving that in the CG-exposed group, p...
Access restricted. Please log in or start a trial to view this content.
Discussion
In this study, a mouse PD model is presented by i.p. injection of CG, and the results showed peritoneal fibrosis and functional deterioration in this model, which mimicked the PD patient's condition.
There are several critical steps in the protocol. First, for performing an i.p. injection of CG or NS, the abdominal wall skin of the mouse must be picked up using forceps to prevent puncture-induced intraperitoneal organ damage. Second, while collecting the peritoneum of the abdominal wall fo...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We sincerely thank Shin-Han Tseng for the critical discussion and partial execution of the study. This study was supported by EDAHP110003 and NCKUEDA110002 from the Research Foundation of E-DA Hospital and the National Cheng Kung University, Taiwan.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 0.9% Normal Saline | Y F CHEMICAL CORP., New Taipei City, Taiwan | - | |
| 10% neutral buffered formalin | Taiwan Burnett International Co., Ltd., Taipei City, Taiwan | 00002A | |
| Automatic biochemical analyzer | Hitachi Ltd., Tokyo, Japan | Labospect Series 008 | for determining glucose concentration |
| Chlorhexidine digluconate solution, 20% in H2O | Sigma-Aldrich, MO, USA | C9394 | diluted to 0.1% with 15% ethanol for injection |
| Ethanol | Avantor Performance Materials, LLC, PA, USA | BAKR8006-05 | diluted to 15% with normal saline for working concentration |
| Glucose (Dianeal) | Baxter International, Inc., IL, USA | FNB9896 | Commercial dialysis solution (4.25%) |
| GraphPad Prism 8.0 | GraphPad Software, Inc., CA, US | ||
| L-type Glu 2 assay | FUJIFILM Wako, Japan | 461-32403 | |
| Xylazine 20 | Juily Pharmaceutical Co., Ltd., New Taipei City, Taiwan | - | |
| Zoletil 50 | Virbac Laboratories, Carros, France | - |
References
- Han, S. H., et al. Improving outcome of CAPD: twenty-five years' experience in a single Korean center. Peritoneal Dialysis International. 27 (4), 432-440 (2007).
- Kawaguchi, Y., Hasegawa, T., Nakayama, M., Kubo, H., Shigematu, T. Issues affecting the longevity of the continuous peritoneal dialysis therapy. Kidney International Supplements. 62, 105-107 (1997).
- Lee, Y. C., et al. Vitamin D can ameliorate chlorhexidine gluconate-induced peritoneal fibrosis and functional deterioration through the inhibition of epithelial-to-mesenchymal transition of mesothelial cells. BioMed Research International. 2015, 595030(2015).
- Nakamoto, H., Kawaguchi, Y., Suzuki, H. Is technique survival on peritoneal dialysis better in Japan. Peritoneal Dialysis International. 26 (2), 136-143 (2006).
- Schaefer, F., Klaus, G., Muller-Wiefel, D. E., Mehls, O. Current practice of peritoneal dialysis in children: results of a longitudinal survey. Mid European Pediatric Peritoneal Dialysis Study Group (MEPPS). Peritoneal Dialysis International. 19, Suppl 2 445-449 (1999).
- Woodrow, G., Turney, J. H., Brownjohn, A. M. Technique failure in peritoneal dialysis and its impact on patient survival. Peritoneal Dialysis International. 17 (4), 360-364 (1997).
- Schmidt, D. W., Flessner, M. F. Pathogenesis and treatment of encapsulating peritoneal sclerosis: basic and translational research. Peritoneal Dialysis International. 28, Suppl 5 10-15 (2008).
- Augustine, T., Brown, P. W., Davies, S. D., Summers, A. M., Wilkie, M. E. Encapsulating peritoneal sclerosis: clinical significance and implications. Nephron Clinical Practice. 111 (2), 149-154 (2009).
- Di Paolo, N., Nicolai, G. A., Garosi, G. The peritoneum: from histological studies to mesothelial transplant through animal experimentation. Peritoneal Dialysis International. 28, Suppl 5 5-9 (2008).
- Fusshoeller, A. Histomorphological and functional changes of the peritoneal membrane during long-term peritoneal dialysis. Pediatric Nephrology. 23 (1), 19-25 (2008).
- Goffin, E. Peritoneal membrane structural and functional changes during peritoneal dialysis. Seminars in Dialysis. 21 (3), 258-265 (2008).
- Ito, T., Yorioka, N. Peritoneal damage by peritoneal dialysis solutions. Clinical and Experimental Nephrology. 12 (4), 243-249 (2008).
- Io, K., et al. SAHA suppresses peritoneal fibrosis in mice. Peritoneal Dialysis International. 35 (3), 246-258 (2015).
- Yoh, K., Ojima, M., Takahashi, S. Th2-biased GATA-3 transgenic mice developed severe experimental peritoneal fibrosis compared with Th1-biased T-bet and Th17-biased RORgammat transgenic mice. Experimental Animals. 64 (4), 353-362 (2015).
- Karl, Z. J. T., et al. Peritoneal Equilibration Test. Peritoneal Dialysis International. 7 (3), 138-148 (1987).
- Lee, Y. C., et al. The clinical implication of vitamin D nanomedicine for peritoneal dialysis-related peritoneal damage. International Journal of Nanomedicine. 14, 9665-9675 (2019).
- Goldner, J. A. Modification of the masson trichrome technique for routine laboratory purposes. The American Journal of Pathology. 14 (2), 237-243 (1938).
- Cheng, F. Y., et al. Novel application of magnetite nanoparticle-mediated vitamin D3 delivery for peritoneal dialysis-related peritoneal damage. International Journal of Nanomedicine. 16, 2137-2146 (2021).
- Ross, A., Willson, V. L. Basic and Advanced Statistical Tests: Writing Results Sections and Creating Tables and Figures. , SensePublishers. 13-16 (2017).
- Suga, H., et al. Preventive effect of pirfenidone against experimental sclerosing peritonitis in rats. Experimental and Toxicologic Pathology. 47 (4), 287-291 (1995).
- Ishii, Y., et al. An experimental sclerosing encapsulating peritonitis model in mice. Nephrology Dialysis Transplantation. 16 (6), 1262-1266 (2001).
- Nishino, T., et al. Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress peritoneal fibrosis in rats. Kidney International. 64 (3), 887-896 (2003).
- Mishima, Y., et al. Enhanced expression of heat shock protein 47 in rat model of peritoneal fibrosis. Peritoneal Dialysis International. 23 (1), 14-22 (2003).
- Kushiyama, T., et al. Effects of liposome-encapsulated clodronate on chlorhexidine gluconate-induced peritoneal fibrosis in rats. Nephrology Dialysis Transplantation. 26 (10), 3143-3154 (2011).
- Nishino, T., et al. Involvement of lymphocyte infiltration in the progression of mouse peritoneal fibrosis model. Renal Failure. 34 (6), 760-766 (2012).
- Lua, I., Li, Y., Pappoe, L. S., Asahina, K. Myofibroblastic conversion and regeneration of mesothelial cells in peritoneal and liver fibrosis. The American Journal of Pathology. 185 (12), 3258-3273 (2015).
- Kitamura, M., et al. Epigallocatechin gallate suppresses peritoneal fibrosis in mice. Chemico-Biological Interactions. 195 (1), 95-104 (2012).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved