Method Article
Transplantation of Human Stem Cell-Derived GABAergic Neurons into the Early Postnatal Mouse Hippocampus to Mitigate Neurodevelopmental Disorders
In This Article
Summary
Transplantation of human pluripotent stem cell-derived GABAergic neurons generated by neuronal programming could be a potential treatment approach for neurodevelopmental disorders. This protocol describes the generation and transplantation of human stem cell-derived GABAergic neuronal precursors into the brains of neonatal mice, allowing long-term investigation of grafted neurons and evaluation of their therapeutic potential.
Abstract
A reduced number or dysfunction of inhibitory interneurons is a common contributor to neurodevelopmental disorders. Therefore, cell therapy using interneurons to replace or mitigate the effects of altered neuronal circuits is an attractive therapeutic avenue. To this end, more knowledge is needed about how human stem cell-derived GABAergic interneuron-like cells (hdINs) mature, integrate, and function over time in the host circuitry. Of particular importance in neurodevelopmental disorders is a better understanding of whether these processes in transplanted cells are affected by an evolving and maturing host brain. The present protocol describes a fast and highly efficient generation of hdINs from human embryonic stem cells based on the transgenic expression of the transcription factors Ascl1 and Dlx2. These neuronal precursors are transplanted unilaterally, after 7 days in vitro, to the hippocampus of neonatal 2-day-old mice. The transplanted neurons disperse in the ipsi- and contralateral hippocampus of a mouse model of cortical dysplasia-focal epilepsy syndrome and survive for up to 9 months after transplantation. This approach allows for investigating the cellular identity, integration, functionality, and therapeutic potential of transplanted interneurons over an extended time in developing healthy and diseased brains.
Introduction
The establishment, maturation, and refinement of neuronal networks occur during the perinatal and early postnatal period and represe a crucial time windows for brain development1. From an after-birth exuberance of connectivity, the brain evolves to a fine-tuning of connections that extend until adolescence2. Consequently, alterations in genes expressed during these periods, as well as external factors or insults, define an individual's predisposition to multiple neurodevelopmental disorders. Impairments in cognition and motor function unfold over time, and pharmacological treatments are limited, the majority targeting symptoms with the risk of severe side effects.
Dysfunction in gamma-aminobutyric acid (GABA)ergic inhibition has been shown to be a major contributor to the underlying cause of various neurodevelopmental disorders3, such as fragile X syndrome, Angelman syndrome, epilepsy, schizophrenia, and autism. GABA is the major inhibitory transmitter of the central nervous system and is instrumental for maintaining excitatory/inhibitory (E/I) balance, the synchronization of neuronal firing, and computation. GABAergic interneurons are a heterogeneous population of neurons, with increasing functional intricacy in more complex brain regions4 and with evolution5,6. Considering the limited endogenous regenerative capacity of the human brain and the implication of interneuron dysfunction in several neurological disorders, the transplantation of GABAergic interneurons may be a promising therapeutic avenue to explore. Along this line, human stem cell-derived GABAergic interneuron-like cells (hdINs) seem to be the most translational and viable source for this purpose compared to rodent allogenic neuronal precursors or other sources used elsewhere7. Protocols for generating GABAergic neurons from diverse cell sources are available8,9,10,11,12,13, but more knowledge is required on how hdINs mature, integrate, and function over time in a developing pathologic brain. Several studies have identified alterations in genes active during cortical patterning, establishing neuronal connectivity14, and tuning physiological E/I balance15. Neonatal transplantation of hdINs into mouse models with corresponding genetic perturbations allows us to follow the interplay between host and graft, which is knowledge necessary to determine potential therapeutic strategies.
Immunomodulation is commonly and successfully used in xenograft transplantations to avoid triggering a host immune response and rejection16. However, the administration of immunosuppressive drugs, such as cyclosporine A, causes renal toxicity after chronic administration, is labor-intensive because of the need for daily intraperitoneal injections to achieve stable systemic concentrations, causing animal stress17, and has off-target effects that can interact with pathology18. In addition, compromising the immune system has been shown to change behavioral phenotypes19, with alterations in the corresponding neuroanatomical regions20. Transplantation in the first week of life has been shown to allow for adaptation to transplanted cells21,22, while others have reported initial survival followed by rejection of the grafts within the first postnatal month23,24.
This protocol describes procedures from hdIN generation to cell transplantation in neonatal mice resulting in long-term graft survival and allowing for the investigation of the neuronal specificity, synaptic integration, function, and therapeutic potential of transplanted human interneurons during physiological and pathological development.
Protocol
All experimental procedures were approved by the Malmö/Lund Animal Research Ethics Board, ethical permit number 12548-19, and conducted in agreement with the Swedish Animal Welfare Agency regulations and the EU Directive 2010/63/EU for animal experiments. C57BL6/J and Contactin-associated protein-like 2 (Cntnap2) knock-out (KO) mice, both males and females, were used at postnatal day (P) 2 for the present study. Human embryonic stem cells (hESCs) were used. The animals and stem cells were obtained from commercial sources (see Table of Materials).
1. Generation of the hdIN precursors
NOTE: All the steps in this section are done in a cell culture hood. The hESCs were maintained as feeder-free cells on coated plates using a stem cell culture medium and passaged as colonies.
- Perform forward programming of the hESCs to hdIN precursors (Figure 1).
NOTE: The forward programming approach is based on the procedure described in Gonzalez-Ramos et al.8 and Yang et al.9.- Transduce the hESCs with lentiviral vectors containing the Tet-On system in a fresh stem cell culture medium containing 10 µM of ROCK inhibitor (RI) (see Table of Materials). Keep at 37 °C in the incubator.
- Change the medium to a fresh stem cell culture medium 16 h after transduction and maintain the transduced cells in the same way as the non-transduced hESCs.
- When confluent, detach the hESCs as single cells using a cell detachment solution (see Table of Materials) and plate them in coated 6-well plates at a density of 3 x 105 cells/well in stem cell culture medium containing 10 µM of RI on day in vitro (DIV) −1.
- At 1 DIV, replace the culturing medium with N2 medium containing 2 g/L of doxycycline (DOX, see Table of Materials).
NOTE: DOX is used to induce transgene expression of Ascl1 and Dlx29, from 1 DIV to 7 DIV, and then continues in vivo by its addition to the drinking water25. - At 2 DIV, an antibiotic resistance selection period starts that will last until 5 DIV. Add puromycin (puro) and hygromycin (hygro) to the fresh medium (see Table of Materials). Change the medium on 2 DIV, 4 DIV, and 5 DIV.
NOTE: Optimize the concentrations of puro and hygro before the differentiation protocol using transduced and non-transduced hESCs to ensure the survival of only the cells carrying the antibiotic resistance cassettes. In this protocol, 0.5 µg/mL of puro and 750 µg/mL of hygro were used. - At 5 DIV, the antibiotic selection period ends. Change to N2 medium supplemented with 2 g/L of DOX and 4 µM of cytosine β-D-arabinofuranoside (Ara-C, see Table of Materials).
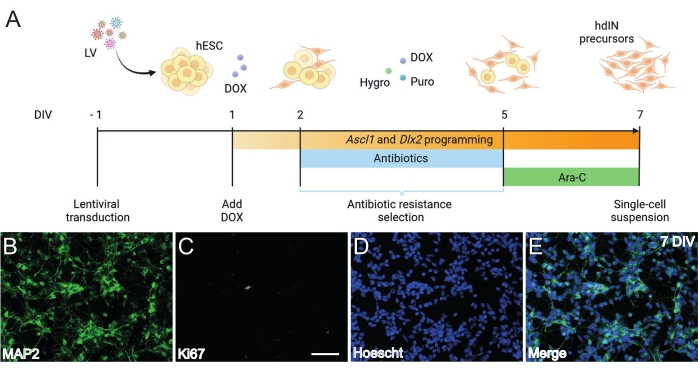
Figure 1: Generation of hdIN precursors from hESCs by overexpressing Ascl1 and Dlx2. (A) Schematics of the differentiation protocol used for the generation of hdIN precursors. (B-E) Immunocytochemistry of hdIN precursors at 7 DIV for (B) the neuronal marker MAP2, (C) the proliferative marker Ki67, (D) general nuclear staining, and (E) a merge of the previous markers. Scale bar: 50 µm. Please click here to view a larger version of this figure.
2. Preparation of the single-cell suspension for transplantation
NOTE: All the steps in this section are done in the cell culture hood. On 7 DIV, hdIN precursors are dissociated and used for transplantation.
- Perform detachment of the cells following the steps below.
- Remove the medium and carefully rinse the cells with DPBS without calcium and magnesium.
- Add 400 µL of the cell detachment solution (see Table of Materials) to each well of the 6-well plate.
NOTE: Ensure the coverage of the whole surface by the solution. - Incubate for 2-3 min at 37 °C in the incubator until the border of the cells starts to look shiny, indicating enzymatic degradation of cell surface proteins and detachment from the well surface.
NOTE: To increase cell survival, do not wait too long when all the cells are completely detached and floating around. - Then, add 600 µL of fresh N2 medium to each well of the 6-well plate to stop the enzyme, and mechanically with the pipette, help to detach the cells to obtain a single-cell suspension.
- Transfer the cell suspension to a plastic tube (15 mL) and centrifuge at 180 x g for 4 min at room temperature (RT).
- Perform resuspension of the cells.
- Discard the supernatant using a vacuum system (carefully, without disturbing the pellet) and resuspend the pellet in the transplantation medium containing 10 µM of RI, 1 µg/mL of DNase, and 2 µg/µL of DOX.
- Count the total cells in the suspension using a counting chamber and a manual cell counter and adjust the volume to a final concentration of 100,000 cells/µL.
- Keep the cell suspension in a closed tube on ice until transplantation for a maximum of 4 h.
NOTE: The transplantation has to be performed on the same day as the preparation of the cell suspension (7 DIV).
3. Intrahippocampal cell transplantation
NOTE: All the steps in this section are performed outside the cell culture hood in the animal facility. Early postnatal transplantation of cells into the brain was performed on P2, considering P0 the day of birth.
- Prepare for the transplantation experiment following the steps below.
- Autoclave all the surgical material or, if not possible, sterilize with another approved method.
- Mount the injecting syringe in the holder without any glass capillary. Rinse the needle with water.
NOTE: The needle must be 33 G. - Curve 90° the tip of a 30 G insulin needle.
- Create a homemade Play-Doh-like stage for positioning the pup with the head flat (Figure 2A).
- Fetch the mice cage with the mother and pups before preparing anything for the surgery to allow them to acclimatize to the new environment.
- Prepare a tray with wet ice and an empty cage with some paper for the isoflurane.
- Anesthetize the mouse pup.
- Soak a piece of paper with isoflurane (see Table of Materials) and place it inside the empty cage. Carefully take one pup and place it into the cage with the paper soaked in isoflurane.
NOTE: Never leave less than three pups with the mum. Do not exceed 20-30 s of isoflurane anesthesia, or the pup may die. - Verify the effect of the anesthesia by movement cessation.
- Immediately after anesthesia, place the pup onto a wet tissue on the surface of wet ice. Keep the animal on ice for 3 min until the upper limbs become whiteish (Figure 2B, blue arrow). This usually takes 2-3 min.
- Use this time for loading the syringe with the cell suspension. Resuspend the cells carefully with a pipette before.
- Place the pup on the stereotaxic frame. Use the ear bars in the opposite direction (Figure 2A, magenta arrows).
NOTE: The back side of the ear bars is less pointy, and as P2 pups do not have ears, use the flatter side to avoid hurting the animal. - From the side, check if the head is straight. The head must be flat; use the Play-Doh-like stage to adjust for that.
NOTE: Pups at this age have not yet opened their eyes, so no additional step in this regard needs to be done. Pups do not have fur at this age, so no shaving of the area is required. No specific pain treatment is given as it is not an open incision to the skin, and no suturing is involved. - Keep the pup covered on ice (on top of tissue paper) for the entire duration of the surgery.
NOTE: Do not place the ice in direct contact with the pup's skin.
- Soak a piece of paper with isoflurane (see Table of Materials) and place it inside the empty cage. Carefully take one pup and place it into the cage with the paper soaked in isoflurane.
- Perform the injection.
- Clean the surface of the skin using a soft tissue soaked in ethanol.
- Identify lambda and set the coordinates to zero on the digital display console of the stereotaxic instrument (Figure 2C, yellow dashed line). Sagittal and lambdoid sutures are easily visible by the eye, since they are vascularized, as red lines.
NOTE: If experiencing difficulties visualizing lambda, place a small piece of ice on the skin and wait a couple of minutes for it to cool down. Then, remove the piece of ice, and the subtle whitening of the skin will allow you to visualize. - Relocate the injection needle to the desired coordinates. In the described protocol, the targeted region was the hippocampus, and the coordinates were as follows: anterior-posterior (AP) +0.85 and medio-lateral (ML) +1.35.
- Use a 90° bent insulin needle to penetrate the skull and create a tiny hole.
- Bring down the injection needle until it has crossed the skull, and zero the dorso-ventral (DV) coordinates. Lower the needle until the desired DV coordinates. In the described protocol, the coordinates were DV −1.1.
- Inject according to the predetermined time intervals.
NOTE: In the described protocol, the exact timings were the following: (i) after going down to the DV coordinate, wait 3 min, (ii) inject 1 µL of volume for 5 min, and (iii) after all the volume has been injected, wait 3 min. - Retract the needle slowly.
- End the procedure.
NOTE: The surgery cannot last more than 15 min to ensure the pup's survival and avoid any damage derived from the anesthesia by hypothermia.- Warm up the pup with the hands until it starts moving before giving it back to the mother.
- Give DOX at a concentration of 1 mg/mL in 0.5% sucrose solution as drinking water for at least 2 days before and 3 weeks post transplantation (PT) to continue the cell differentiation in vivo.
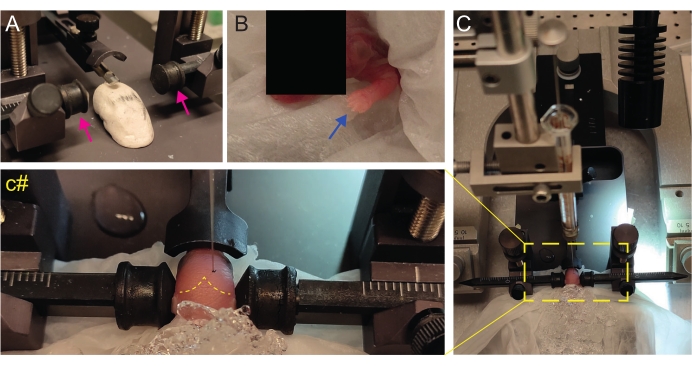
Figure 2: Stereotaxic transplantation in newborn mice pups at P2. (A) A Play-Doh-like stage for holding the pup's body in position and inverted ear bars (magenta arrows). (B) White front paw (blue arrow) indicative of the reduced blood flow in that area so that the pup is experiencing anesthesia by hypothermia. (C) Overview of the setup with the pup already covered by ice over the soft tissue paper. (c#) Closed-zoom of the head of the pup, with the injection needle already inserted into the brain (yellow dashed line indicating lambda and lambdoid sutures). This figure is adapted from Gonzalez Ramos et al.27. Please click here to view a larger version of this figure.
Results
Following the protocol presented here and illustrated in Figure 1A, hdIN precursors were not proliferative yet at 7 DIV as defined by (i) negative immunoreactivity for the cell cycle marker Ki67 and (ii) expressing neuronal markers such as microtubule-associated protein 2 (MAP2) (Figure 1B-E). This characterization was performed on leftover cells replated for 24 h after having undergone all the procedure steps. In addition, the gene expression analysis published previously indicated that a rapid transition from the pluripotent state to a neuronal phenotype occurs around 4 DIV and 7 DIV8. Overall, these results confirmed the presence of postmitotic cells and the absence of risk for teratoma formation.
Next, the hdIN precursors' survival after early postnatal transplantation into the hippocampus of wild-type (WT) mice was tested by immunohistochemistry against the human cytoplasmatic marker STEM121. The hdIN precursors were transplanted into the right dorsal hippocampus of naïve immunocompetent mice at P2, which were then sacrificed at P14 and 2 months PT. Grafted cells were found across the whole dorsal hippocampus, as well as dispersed through the corpus callosum and the contralateral hippocampus, at both time points. Moreover, at both time points, grafted hdINs expressed Ascl1, one of the induction transcription factors (Supplementary Figure 1), and were not proliferative, as indicated by the absence of Ki67 expression (Supplementary Figure 2).
Importantly, no immune reaction or local inflammation against the transplanted cells was found either at P14 or 2 months PT, as assessed by the absence of reactive microglia identified using Iba1, CD68, and galectin-3 (Gal3) (Figure 3), the extent of astrogliosis determined by the glial fibrillary acidic protein (GFAP) and inflammatory cytokines such as interleukine-1 (IL-1), and the absence of cytotoxic T lymphocytes (CD8) (Figure 4).
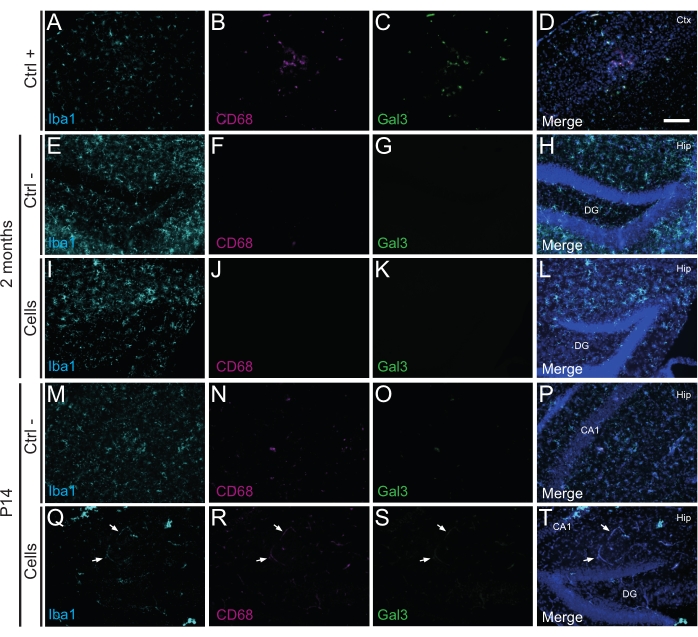
Figure 3: hdINs at P14 and 2 months PT into the hippocampus of newborn WT mice without triggering immune rejection from the host tissue. Immunofluorescence for Iba1, CD68, and Gal3 markers in brain tissue from (A-D) the proximity of an ischemic core area in an electrocoagulation stroke mouse model (positive control, Ctrl +), (E-H) negative control animals (Ctrl-) at 2 months and (M-P) P14, and (I-L) animals that have undergone cell transplantation at 2 months and (Q-T) P14. The white arrows indicate some examples of blood vessels visible at all channels due to autofluorescence. Abbreviations: Ctx = cortex; Hip = hippocampus; DG = dentate gyrus; CA1 = cornu ammonis 1. Scale bar: 50 µm. Please click here to view a larger version of this figure.
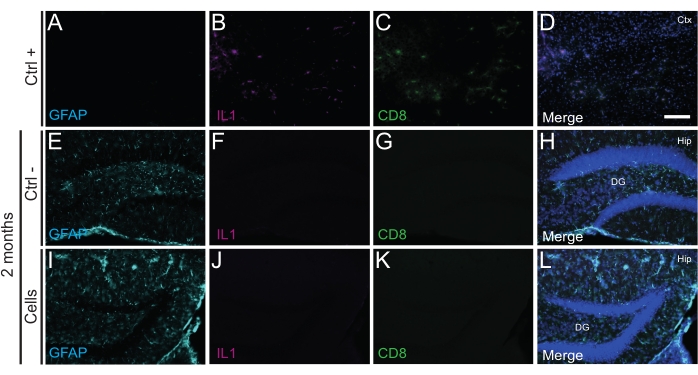
Figure 4: hdINs at 2 months PT into the hippocampus of newborn WT mice without triggering immune rejection from the host tissue. Immunofluorescence for IL1, GFAP, and CD8 markers in brain tissue from (A-D) the proximity of an ischemic core area in an electrocoagulation stroke mouse model (positive control, Ctrl +), (E-H) negative control animals (Ctrl-) at 2 months, and (I-L)animals that have undergone cell transplantation at 2 months. Abbreviations: Ctx = cortex; Hip = hippocampus; DG = dentate gyrus. Scale bar: 50 µm. Please click here to view a larger version of this figure.
Similarly, hdIN precursors were also transplanted into the hippocampus of Cntnap2 KO mice, a model for autism spectrum disorder and cortical dysplasia-focal epilepsy syndrome. In the Cntnap2 KO mice, indeed, the hdINs survived up to 9 months PT and were localized at the injection site, although they were also dispersed across the ipsilateral and even the contralateral hippocampus as observed in the WT mice (Figure 5). Moreover, most of the grafted hdINs were immunoreactive for interneuron markers, as expected from previous results in vitro8,26 and in adult rodents in vivo25.
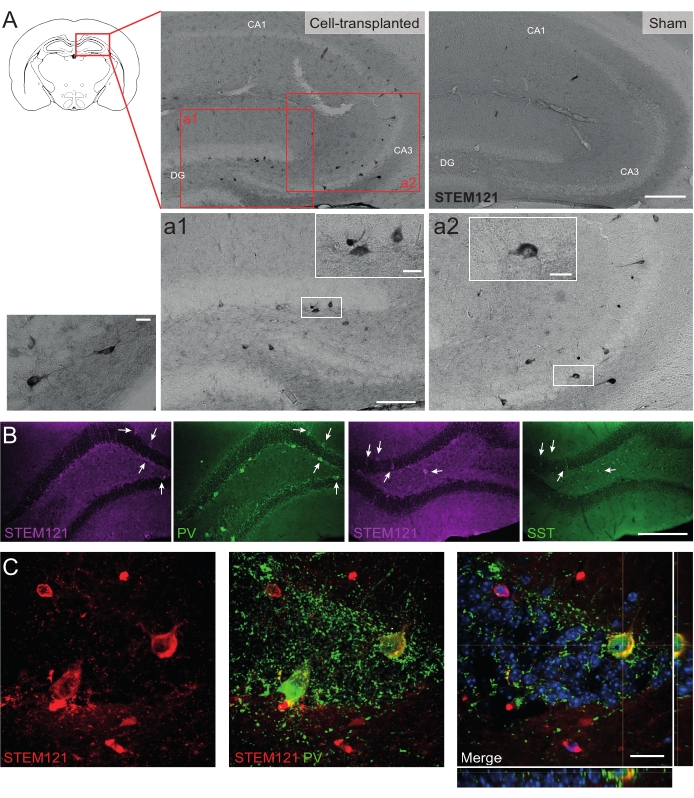
Figure 5: Grafted hdINs in the hippocampus of Cntnap2 KO mice at 9 months PT. (A) Immunochemistry against the cytoplasmatic human marker STEM121 in cell-transplanted (left) and sham (right) mice. (a1 and a2) Magnified images of STEM121 positive cells. (B) Immunofluorescence for STEM121 (magenta) and the interneuron markers parvalbumin (PV) and somatostatin (SST). White arrows indicate double positive cells for STEM121 and the respective interneuron marker. (C) Orthogonal view of a grafted hdIN immunoreactive for STEM121 and PV. Scale bar: 200 µm (A and B), 100 µm (a1 and a2), 20 µm (small square magnification in a1 and a2, and C). Please click here to view a larger version of this figure.
Supplementary Figure 1: Grafted hdINs at 2 months PT into the hippocampus of newborn WT mice expressing Ascl1. Immunofluorescence against Ascl1 and the cytoplasmatic human marker STEM121 at the (A) CA3 and (B) DG in cell-transplanted WT mice. (a) Magnified image of a STEM121 positive cell. The white arrows indicate double-positive cells for STEM121 and Ascl1. Scale bar: 100 µm. Please click here to download this File.
Supplementary Figure 2: Post-mitotic grafted hdINs at 2 months PT into the hippocampus of newborn WT mice. Immunofluorescence against the proliferative marker Ki67 and the cytoplasmatic human marker STEM121 in (A) naïve and (B) cell-transplanted WT mice. (b) Magnified image of a STEM121 positive cell. The yellow arrow indicates a cell positive for Ki67 and negative for STEM121. The white arrow indicates cells positive for STEM121 and negative for Ki67. The white asterisk points out a lateral ventricle. Scale bar: 100 µm. Please click here to download this File.
Discussion
The present protocol describes a robust, fast, simple, and widely accessible methodology to generate hdIN precursors in vitro and its use as early interventional cell therapy in preclinical models of neurodevelopmental disorders.
Even though some of the characteristic phenotypes of neurodevelopmental disorders arise during adolescence or adulthood, pathophysiological alterations are already present during early development. For this reason, early intervention would be highly warranted for achieving beneficial effects by acting in critical brain developmental periods before symptomatology or clinical manifestation. In the future, genetic screening and the development of biomarkers will afford prophylactic or pre-symptomatic treatment, representing a game changer for those patients. Therefore, hdIN precursors were transplanted early after birth in the Cntnap2 KO mouse model when epileptogenic changes in the neuronal network might be ongoing28 and at a timepoint at which cellular alterations have been described in this animal model29. It is important, however, to consider the potential pitfalls of age extrapolation and the timing of certain processes in the mouse versus the human brain.
Focusing on the procedure itself, the differentiation protocol presented here is based on the use of transcription factors, which allow for fast and highly efficient programming of stem cells compared with other protocols elsewhere based on small molecules10,30. A potential drawback of this approach could be the requirement for lentiviral vectors, which carries a risk of insertional mutagenesis. Two critical steps in the protocol are the addition of the antibiotics and the anti-mitotic agent to the medium to select for cells expressing the transcription factors and eliminate proliferative cells, avoiding the risk of teratoma formation, respectively. Although only hdIN precursors were tested in this study, the procedure is expected to be feasible with other cell sources and programming/differentiation protocols. Nevertheless, other neuronal subtypes and/or models should be validated.
The hdIN precursor's age for transplantation, 7 DIV, was decided based on (i) the absence of proliferative cells, assessed by immunoreactivity against Ki67, (ii) together with the previously reported observation of decreases in the expression of pluripotency genes such as POU5F1 and the appearance of the neuronal marker MAP2 and the interneuron marker GAD1 at that timepoint8. However, the original work describing this protocol performed transplantations at 14 DIV after DOX withdrawal9. This raises questions about whether DOX in the mother's drinking water can reach cells grafted into the brains of nursing pups via the milk, or if 7 DIV of DOX induction is enough to establish the GABAergic fate. Although Yang et al. identified 14 days of DOX as sufficient to generate stable neuronal cells in vitro9, Gonzalez-Ramos et al.8 detected GAD1 gene expression already at 7 DIV, indicating that the downstream activation of GAD67 by Ascl1 and Dlx2 has already occurred at this time point. Hence, patterning has begun at 7 DIV and might be less dependent on the DOX treatment. Moreover, evidence in rodents and humans indicates the presence of DOX in breast milk31,32, and the results presented here show that grafted hdINs were immunoreactive for Ascl1 at 2 weeks and 2 months PT and interneuron markers later on at 9 months PT. Within the grafted population, besides PV and SST positive neurons, other markers for subpopulations of interneurons were also found in lower amounts, such as calretinin (CR) and calbindin (CB).
A challenging aspect of this procedure is the coordination of the timings for both differentiation and the age of the pups. Usually, mouse gestation takes 21 days after setting up the mating cage, albeit this can vary sometimes. This scenario does not occur when performing cell transplantations in adult rodents when everything can be carefully planned and arranged. Nevertheless, this can be easily mitigated by setting up two to three mating cages with a 2 day interval or two to three differentiation batches with a 2 day time-lapse from each other.
Although the mice used in this study were neither immunodeficient nor immunosuppressed, the transplanted cells survived up to 9 months in vivo, and markers of immune reaction against xenogeneic cells or local inflammation were not observed at either P14 or 2 months PT. Immune rejection of grafted xenogeneic cells is triggered against MHC/peptides, and the key cellular mediators of graft rejection are T lymphocytes and microglial cells33,34. Therefore, immunoreactivity to markers of T cells, as well as reactive microglia, was explored. No signs of immune rejection of the grafted cells in the host tissue were detected either by levels of reactive microglia or by the presence of T lymphocytes in WT mice at P14 or 2 months. Moreover, no local inflammation was observed based on the assessed levels of astrogliosis and inflammatory cytokines. This outcome could partly be dependent on neonatal immune tolerance35,36,37, observed by other cell identities, locations, and animal models35,38. Englund et al. identified regional differences in the outcome of the grafted cells in terms of migration and maturation, including the observation of grafted cells in the adjacent white matter35.
Finally, a greater dispersion of the grafted cells within the hippocampus was observed compared to other studies transplanting into adult rodents, where hdINs remained as a grafted core25. This dispersion also differed from results observed previously by Yang et al.9, which could be explained in this case by the age of the cells at the time of transplantation.
Disclosures
The authors declare no competing interests.
Acknowledgements
This project was funded by the Swedish Research Council (Grant Number: 2016-02605, M.A.), the Swedish Brain Foundation F02021-0369 (M.A.), the Crafoord Foundation (M.A.), and the European Union Horizon 2020 Programme (H2020-MSCA-ITN-2016) under the Marie Skłodowska-Curie Innovative Training Network project Training4CRM No. 722779 (M.K.). We are extremely grateful for the help from Andrés Miguez, from Josep Maria Canals' lab (Laboratory of Stem Cells and Regenerative Medicine, University of Barcelona), for teaching stereotaxic cell transplantation in P2 newborn mice, and Mackenzie Howard, group leader at the University of Texas at Austin, for the advice and preliminary coordinates for cell transplantation into the hippocampus of P2 newborn mice. We thank Susanne Geres for assisting with animal care and Ling Cao for the help with processing tissue, as well as students that have contributed in one way or another to the study and specifically Diana Hatamian. Finally, some of the graphics used to illustrate this paper were created with BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 30 G needle | B Braun | 4656300 | |
| 33 G needle for Hamilton syringe | Hamilton | 7762-06 | |
| 4-well plates | Thermo Scientific | 176740 | |
| Accutase | STEMCELL Technologies | 7920 | Cell detachment solution use for splitting cells (hESC and hdIN precursors) |
| Adjustable volume pipettes 10, 20, 200, 1000 µL | |||
| Alexa Fluor Plus 488/555/647 | Thermo Fisher | 1:1000 | |
| Anti-CD68 (Rat) | Bio-Rad | MCA1957 | 1:200 |
| Anti-CD8 (Rabbit) | Abcam | 203035 | 1:200 |
| Anti-Galectin 3 (Goat) | R&D systems | AF1197 | 1:500 |
| Anti-GFAP (Guiena Pig) | Synaptic systems | 173004 | 1:500 |
| Anti-Iba1 (Rabbit) | WAKO | 19119741 | 1:500 |
| Anti-IL1 (Goat) | Santa Cruz Biotech | SC-106 | 1:400 |
| Anti-Ki67 | Abcam | ab16667 | 1:250 |
| Anti-Ki67 (Rabbit) | Novocastra | NCL-Ki67p | 1:250 |
| Anti-MAP2 (Chicken) | Abcam | ab5392 | 1:2000 |
| Anti-Mash1 (Ascl1) | Abcam | ab74065 | 1:1000 |
| Anti-Parvalbumin (Rabbit) | Swant | PV 27 | 1:5000 |
| Anti-Somatostatin (Rat) | Millipore | MAB354 | 1:150 |
| Anti-STEM121 (Mouse) | Takara Bio | Y40410 | 1:400 |
| Avidin/Biotin Blocking Kit | VECTOR Laboratories | SP-2001 | |
| B6.129(Cg)-Cntnap2tm1Pele/J | Jackson Laboratory | 17482 | Animal model |
| Biotinylated Horse anti-Mouse | VECTOR Laboratories | BA-2001 | 1:200 |
| Burker Chamber | Thermo Fisher Scientific | 10628431 | |
| C57BL/6J | Janvier Labs | Animal model | |
| Centrifuge | For 15 mL tubes | ||
| Confocal microscope | Nikon | Confocal A1RHD microscope | |
| Costar 6-well Clear TC-treated | Corning | 3516 | |
| Cy3 Streptavidin | Jackson ImmunoResearch | 016-160-084 | 1:200 |
| Cytosine β-D-arabinofuranoside (AraC) | Sigma | C1768 | 4 µM |
| DAB Substrate Kit, Peroxidase (With Nickel) | VECTOR Laboratories | SK-4100 | |
| Digital Stereotax | KOPF | Model 940 | |
| DMEM/F12 | Thermo Fisher Scientific | 11320082 | Use for the N2 medium |
| DNase I Solution | STEMCELL Technologies | 7900 | 1 µg/mL |
| Doxycyclin | Sigma-Aldrich | D9891 | 2 µg/mL |
| DPBS -/- | Gibco | 14190144 | |
| Epifluorescence microscope | Olympus | BX51 Microscope | |
| Ethanol | Solveco | 70%, 95%, 99.8% | |
| FUW-rTA | Addgene | 20342 | Lentiviral vector |
| FUW-TetO-Ascl1-T2A-puromycin | Addgene | 97329 | Lentiviral vector |
| FUW-TetO-Dlx2-IRES-hygromycin | Addgene | 97330 | Lentiviral vector |
| H1 (WA01) ESC | WiCell | WA01 | Human embryonic stem cell line under a MTA agreement |
| H2O2 | Sigma-Aldrich | 18304 | |
| Hamilton Syringe | Hamilton | 7634-01 | 5 µL |
| HBSS | Gibco | 14175095 | No calcium, No magnesium - Transplantation medium |
| Hoechst 33342 | Invitrogen | H3570 | 1:1000 |
| Hygromycin B | Gibco (Invitrogen) | 10687010 | |
| Incubator | 5% CO2, 37 °C | ||
| Isoflurane Baxter | Apoteket AB | ||
| Manual cell counter | VWR | 720-1984 | |
| Matrigel hESC-Qualified Matrix, LDEV-free | Corning | 354277 | For the coating |
| Methanol | Merck Millipore | 1060091000 | |
| Microscope Coverslips 24 x 60 mm | Thermo Scientific | BBAD02400500#A113MNZ#0## | |
| Microscope Slides | VWR | 631-1551 | |
| Microscope Software | Olympus | CellSens | |
| Mounting media | Merck | 10981 | PVA-Dabco |
| Mouse adaptor to stereotax | RWD | 68030 | |
| mTeSR1 | STEMCELL Technologies | 85850 | Kit Basal Medium and 5X Supplement - Stem cell culture medium |
| N2 supplement | Gibco | 17502048 | |
| NaOH | Sigma-Aldrich | S8045 | 1M |
| Penicillin-Streptomycin | Sigma-Aldrich | P0781 | |
| Pertex | HistoLab | 811 | |
| Pipet Filler | |||
| Play-Doh | |||
| Puromycin (Dihydrochloride) | Gibco | A1113803 | |
| Round cover glasses thickness No. 1.5H (tol. ± 5 μm) 13 mm Ø | Marienfeld | MARI0117530 | For immunocytochemistry |
| Serum | Thermo Fisher | Goat, Donkey, Horse | |
| Sterile pipette tips | For volumes 0.1-1000 µL | ||
| Sterile serological pipettes | 5, 10, 25 mL | ||
| Sterile water Braun | B Braun | 3626873 | |
| Sucrose | Sigma-Aldrich | S8501 | For 0.5% Sucrose solution |
| Triton X-100 | Sigma-Aldrich | X100 | |
| Trypan Blue Solution | Gibco | 15250061 | |
| Tubes | Sarstedt | 15 ml, Eppendorf 1.5 mL | |
| Tweezer | VWR | ||
| Ultra pure water | MilliQ Water System | ||
| Xylene | VWR | 28973.363 | |
| Y-27632 (ROCK inhibitor) | STEMCELL Technologies | 72304 | 10 µM |
References
- Kostović, I., Jovanov-Milošević, N. The development of cerebral connections during the first 20-45 weeks' gestation. Seminars in Fetal and Neonatal. 11 (6), 415-422 (2006).
- Innocenti, G. M., Price, D. J. Exuberance in the development of cortical networks. Nature Reviews Neuroscience. 6 (12), 955-965 (2005).
- Tang, X., Jaenisch, R., Sur, M. The role of GABAergic signalling in neurodevelopmental disorders. Nature Reviews Neuroscience. 22 (5), 290-307 (2021).
- Kepecs, A., Fishell, G. Interneuron cell types are fit to function. Nature. 505 (7483), 318-326 (2014).
- DeFelipe, J., Alonso-Nanclares, L., Arellano, J. I. Microstructure of the neocortex: Comparative aspects. Journal of Neurocytology. 31 (3-5), 299-316 (2002).
- Radonjić, N. V., et al. Diversity of cortical interneurons in primates: The role of the dorsal proliferative niche. Cell Reports. 9 (6), 2139-2151 (2014).
- Turner, D. A., Shetty, A. K. Clinical prospects for neural grafting therapy for hippocampal lesions and epilepsy. Neurosurgery. 52 (3), 632-644 (2003).
- Gonzalez-Ramos, A., et al. Human stem cell-derived GABAergic neurons functionally integrate into human neuronal networks. Scientific Reports. 11, 22050 (2021).
- Yang, N., et al. Generation of pure GABAergic neurons by transcription factor programming. Nature Methods. 14 (6), 621-628 (2017).
- Nicholas, C. R., et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 12 (5), 573-586 (2013).
- Yuan, F., et al. Induction of human somatostatin and parvalbumin neurons by expressing a single transcription factor LIM homeobox 6. eLife. 7, 37382 (2018).
- Fandel, T. M., et al. Transplanted human stem cell-derived interneuron precursors mitigate mouse bladder dysfunction and central neuropathic pain after spinal cord injury. Cell Stem Cell. 19 (4), 544-557 (2016).
- Noakes, Z., et al. Human pluripotent stem cell-derived striatal interneurons: Differentiation and maturation in vitro and in the rat brain. Stem Cell Reports. 12 (2), 191-200 (2019).
- Parikshak, N. N., Gandal, M. J., Geschwind, D. H. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nature Reviews Genetics. 16 (8), 441-458 (2015).
- Marín, O. Interneuron dysfunction in psychiatric disorders. Nature Reviews Neuroscience. 13 (2), 107-120 (2012).
- Sloan, D. J., Wood, M. J., Charlton, H. M. The immune response to intracerebral neural grafts. Trends in Neurosciences. 14 (8), 341-346 (1991).
- Diehl, R., et al. Immunosuppression for in vivo research: State-of-the-art protocols and experimental approaches. Cellular & Molecular Immunology. 14 (2), 146-179 (2017).
- Osman, M. M., et al. Cyclosporine-A as a neuroprotective agent against stroke: Its translation from laboratory research to clinical application. Neuropeptides. 45 (6), 359-368 (2011).
- Quinnies, K. M., Cox, K. H., Rissman, E. F. Immune deficiency influences juvenile social behavior and maternal behavior. Behavioral Neuroscience. 129 (3), 331-338 (2015).
- Fernandes, D. J., et al. Mouse models of immune dysfunction: their neuroanatomical differences reflect their anxiety-behavioural phenotype. Molecular Psychiatry. 27 (7), 3047-3055 (2022).
- Lund, R. D., Rao, K., Hankin, M. H., Kunz, H. W., Gill, T. J. Transplantation of retina and visual cortex to rat brains of different ages. Maturation, connection patterns, and immunological consequences. Annals of the New York Academy of Sciences. 495, 227-241 (1987).
- Olsson, M., Bentlage, C., Wictorin, K., Campbell, K., Björklund, A. Extensive migration and target innervation by striatal precursors after grafting into the neonatal striatum. Neuroscience. 79 (1), 57-78 (1997).
- Mattis, V. B., et al. Neonatal immune-tolerance in mice does not prevent xenograft rejection. Experimental Neurology. 254, 90-98 (2014).
- Nato, G., et al. Immune-tolerance to human iPS-derived neural progenitors xenografted into the immature cerebellum is overridden by species-specific differences in differentiation timing. Scientific Reports. 11, 651 (2021).
- Allison, T., et al. Defining the nature of human pluripotent stem cell-derived interneurons via single-cell analysis. Stem Cell Reports. 16 (10), 2548-2564 (2021).
- Waloschková, E., et al. Human stem cell-derived GABAergic interneurons establish efferent synapses onto host neurons in rat epileptic hippocampus and inhibit spontaneous recurrent seizures. International Journal of Molecular Sciences. 22 (24), 13243 (2021).
- Gonzalez Ramos, A. . Enhancing neuronal inhibition by cell and gene therapy as a novel treatment for epilepsy. , (2022).
- Paterno, R., et al. Hippocampal gamma and sharp-wave ripple oscillations are altered in a Cntnap2 mouse model of autism spectrum disorder. Cell Reports. 37 (6), 109970 (2021).
- Penagarikano, O., et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 147 (1), 235-246 (2011).
- Maroof, A. M., et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 12 (5), 559-572 (2013).
- Angeletti, B., et al. An in vivo doxycycline-controlled expression system for functional studies of the retina. Investigative Ophthalmology & Visual Science. 44 (2), 755-760 (2003).
- Doxycycline. Drugs and Lactation Database (LactMed) Available from: https://www.ncbi.nlm.nih.gov/books/NBK500561 (2021)
- Barker, R. A., Widner, H. Immune problems in central nervous system cell therapy. NeuroRX. 1 (4), 472-481 (2004).
- Hoornaert, C. J., et al. Concise review: Innate and adaptive immune recognition of allogeneic and xenogeneic cell transplants in the central nervous system. Stem Cells Translational Medicine. 6 (5), 1434-1441 (2017).
- Englund, U., Fricker-Gates, R. A., Lundberg, C., Bjorklund, A., Wictorin, K. Transplantation of human neural progenitor cells into the neonatal rat brain: Extensive migration and differentiation with long-distance axonal projections. Experimental Neurology. 173 (1), 1-21 (2002).
- Fainstein, N., Ben-Hur, T. Brain region-dependent rejection of neural precursor cell transplants. Frontiers in Molecular Neuroscience. 11, 136 (2018).
- Denham, M., et al. Neurons derived from human embryonic stem cells extend long-distance axonal projections through growth along host white matter tracts after intra-cerebral transplantation. Frontiers in Cellular Neuroscience. 6, 11 (2012).
- Miguez, A., et al. In vivo progressive degeneration of Huntington's disease patient-derived neurons reveals human-specific pathological phenotypes. bioRxiv. , (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved