A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Quantifying the Antifungal Activity of Peptides Against Candida albicans
In This Article
Summary
This protocol describes a method for obtaining quantitative data on the antifungal activity of peptides and other compounds, such as small-molecule antifungal agents, against Candida albicans. Its use of optical density rather than counting colony-forming units to quantify growth inhibition saves time and resources.
Abstract
Traditional methods for performing antifungal susceptibility testing for Candida albicans are time-consuming and lack quantitative results. For example, a common approach relies on plating cells treated with different concentrations of antifungal molecules on agar plates and then counting the colonies to determine the relationship between molecule concentration and growth inhibition. This method requires many plates and substantial time to count the colonies. Another common approach eliminates the plates and counting of colonies by visually inspecting cultures treated with antifungal agents to identify the minimum concentration required to inhibit growth; however, visual inspection produces only qualitative results, and information on growth at subinhibitory concentrations is lost. This protocol describes a method for measuring the susceptibility of C. albicans to antifungal peptides. By relying on optical density measurements of cultures, the method reduces the time and materials needed to obtain quantitative results on culture growth at different peptide concentrations. The incubation of the fungus with peptides is performed in a 96-well plate using an appropriate buffer, with controls representing no growth inhibition and complete growth inhibition. Following the incubation with the peptide, the resulting cell suspensions are diluted to reduce peptide activity and then grown overnight. After overnight growth, the optical density of each well is measured and compared to the positive and negative controls to calculate the resulting growth inhibition at each peptide concentration. The results using this assay are comparable to the results using the traditional method of plating the cultures on agar plates, but this protocol reduces plastic waste and the time spent on counting colonies. Although the applications of this protocol have focused on antifungal peptides, the method will also be applicable to testing other molecules with known or suspected antifungal activity.
Introduction
Candida albicans is a member of the human microbiota that colonizes numerous locations, including the oral cavity, skin, gastrointestinal tract, and vagina1. For patients that are immunocompromised due to diseases such as human immunodeficiency virus (HIV) and immunosuppressive treatments, the colonization of C. albicans may lead to local or systemic candidiasis2,3. The use of currently available small-molecule antifungal therapeutics, such as amphotericin B, azoles, or echinocandins, can be complicated by solubility and toxicity issues and by the resistance of infections to the therapeutics4,5. Due to the limitations of current antifungal agents, researchers are continually searching for new antifungal molecules with activity against C. albicans.
Antimicrobial peptides (AMPs) are a potential alternative to the current small-molecule antifungal agents6,7,8 and are proposed to be less susceptible to the development of resistance compared to small-molecule drugs9. AMPs are a diverse set of peptides, but they are often cationic, with a broad spectrum of activity10,11,12. AMPs with activity against C. albicans include well-known peptides from the histatin and cecropin families13,14,15, along with more recently described peptides like ToAP2, NDBP-5.7, and the histatin 5 variant K11R-K17R16,17. Due to their potential for treating Candida infections, identifying and designing new AMPs that target C. albicans is an important goal for many research groups.
As part of the process to develop effective AMPs (and other antifungal agents) that target C. albicans, in vitro testing is commonly used to identify promising peptides. Methods to test antifungal activity against C. albicans typically involve incubating cells with serial dilutions of the AMPs (in buffer or medium) in 96-well plates. Several methods are available to assess the antifungal activity following incubation. A technique described by the Clinical Laboratory Standards Institute uses a purely visual assessment of the turbidity of the wells to determine the minimum concentration (MIC) for the complete inhibition of growth (at least 50% inhibition for selected antifungal agents, like azoles and echinocandins) and provides no quantification of growth at sub-MIC concentrations18. Another commonly used approach involves quantifying the viability following incubation with AMPs by plating the contents of the wells on agar plates, incubating the plates, and then counting the number of colony-forming units (CFUs) on the plate. This method has been used for evaluating a number of peptides, including histatin 5-based peptides, LL-37, and human lactoferrin19,20,21. This technique requires a relatively large volume of agar and a high number of plates and involves the tedious counting of CFUs on the plates. To obtain more quantitative data while generating less plastic waste and avoiding counting CFUs, the contents of the wells can be used to inoculate fresh medium in another 96-well plate. After incubating the newly inoculated plate, the growth can be quantified by measuring the optical density at 600 nm (OD600) on an absorbance plate reader. This method has been used to determine the antifungal activity of histatin 5 and its degradation fragments and cell-penetrating peptides17,22,23,24,25.
This protocol describes how to test the antifungal activity of peptides and uses the OD600 method to quantify the reduction in viability of C. albicans due to peptides.
Protocol
Approval was obtained from the University of Maryland, College Park, Institutional Biosafety Committee (IBC) for the work with C. albicans in this protocol (PN 274). The C. albicans strain SC5314 (see Table of Materials) was used in the present study; however, any other strain may also be used.
1. Preparation of the buffer, sterile water, and culture medium
- Prepare sterile 0.1 M sodium phosphate buffer (NaPB)26 at pH 7.4, and dilute to 2 mM and 1 mM with sterile water. Preparing 100 mL each of 2 mM NaPB and 1 mM NaPB will be more than sufficient for most applications of this protocol.
- Prepare sterile liquid yeast-peptone-dextrose (YPD, see Table of Materials) medium (10 g/L yeast extract, 20 g/L dextrose, 20 g/L peptone). Preparing 100 mL of YPD will be more than sufficient for most applications of this protocol.
- Prepare sterile ultrapure water. Preparing 100 mL of water will be more than sufficient for most applications of this protocol.
NOTE: The buffers, media, and water are sterilized by autoclaving at 121 °C on a liquid cycle for an appropriate time based on the volume in the containers being autoclaved27. For example, for bottles containing 500 mL, the exposure time should be 40 min.
2. Inoculation, culturing, and subculturing of C. albicans
CAUTION: Follow all institutional and governmental regulations for working with C. albicans, which is often classified by academic research institutions as a biosafety level (BSL) 2 organism regardless of drug resistance and by the American Type Culture Collection (ATCC) as a BSL1 or BSL2 organism, depending on the drug resistance of the strain28.
NOTE: Perform the inoculation and culturing portions of this step (steps 2.1-2.2) on the day before beginning the testing for antifungal activity. If possible, perform all the protocol steps with the cells (and the solutions that will be incubated with the cells) in a biosafety cabinet.
- Inoculate the desired C. albicans strain into 10 mL of YPD medium in a culture tube.
NOTE: The culture can be inoculated from a freezer stock or from an agar plate, but a consistent source must be used for all data that will be compared. - Grow the culture overnight (~12-16 h) at 30 °C and 230 rpm on a rotary shaker.
- Subculture the overnight culture of C. albicans, and grow to an OD600 of ~1.0-1.2.
- Measure the OD600 of the overnight culture using a UV spectrophotometer (see Table of Materials).
- Based on the OD600 of the overnight culture, use the overnight culture to inoculate a subculture at an OD600 of 0.1 in 10 mL of YPD.
- Grow the subculture at 30 °C on a rotary shaker at 230 rpm until the OD600 reaches ~1.0-1.2, which is likely to take 4-6 h. Complete step 3 (preparation of peptide solutions) while the subculture is growing; the subculture will be diluted for use in the assay in step 4.
3. Preparation of the peptide solutions in a 96-well plate
NOTE: This step may be done ahead of time, if the peptide stock solutions are stable during storage. Typically, the peptide solutions are stored at −20 °C until use. They can be thawed in a room temperature water bath before proceeding to the next step.
- Determine the highest concentration of each peptide to be tested in the assay. This will vary based on the peptides selected. For the data presented in the representative results section, histatin 5 and engineered analogs22 were tested, and the highest concentration tested was 50 µM.
NOTE: The highest concentration to be tested is determined using data reported in the literature or by performing preliminary experiments over a broad range of peptide concentrations. When possible, the highest concentration should be selected to observe a plateau for a complete reduction in viability at the highest concentrations tested and a plateau for no reduction in viability at the lowest concentrations tested, allowing the quantification of the full range of peptide activity. - Dissolve each peptide in sterile water at twice the desired highest concentration. For the data presented in the representative results section, 150 µL of 100 µM solutions of histatin 5 and engineered analogs were prepared in sterile water.
NOTE: If a peptide is not soluble in water, solubilization in another solvent may be necessary prior to this step. Dimethyl sulfoxide (DMSO) is commonly used to solubilize peptides at a high concentration before diluting to the working concentration. Ensure that the final concentration of the DMSO is 1% (v/v) or lower29 and that alternative solvents do not affect the growth of the cells. Also, ensure that the solvent concentration is kept constant in all the wells in steps 3.4-3.5. - Add 40 µL of the desired peptide stock solutions to the first well (Column 1) of each row in a 96-well, round-bottomed culture plate (see Table of Materials). This plate is Plate 1 (Figure 1).
NOTE: Each plate has eight rows that are used for testing peptides. These rows can be used for different peptides or for replicates of the same peptides. Include only a single peptide in each row. - Add 20 µL of sterile ultrapure water to Column 2 to Column 12 of the rows containing peptide (Figure 1).
NOTE: If the peptide stocks were prepared with a solvent other than pure water in step 3.2, the sterile water in this step should be replaced with the solvent present in the peptide stock added to the first column. For example, if the peptide was solubilized in DMSO and the peptide stock contains 1% (v/v) DMSO in water, water containing 1% DMSO must be added to Column 2 to Column 12. - Serially dilute the peptide stock solutions across the plate to Column 10 (Figure 1).
NOTE: Rather than performing the serial dilutions in the plate as described below, the dilutions could also be performed in microcentrifuge tubes and transferred to the 96-well plate.- Remove 20 µL from Column 1, transfer it to Column 2, and mix by pipetting up and down. Column 2 now contains 40 µL of peptide solution at a 1:2 dilution of the concentration in Column 1.
- Remove 20 µL from Column 2, transfer it to Column 3, and mix by pipetting up and down, so Column 3 now contains 40 µL of peptide solution at a 1:4 dilution of the concentration in Column 1.
- Repeat this process until Column 10 contains 40 µL of peptide solution at a 1:512 dilution of the concentration in Column 1.
- Remove 20 µL of peptide solution from Column 10 and discard it. Each column now contains 20 µL of peptide solution (Columns 1-10) or water (Column 11 and Column 12).
NOTE: The wells in Column 11 serve as sterility controls, and the wells in Column 12 serve as controls containing no peptide.
4. Dilution of the C. albicans subculture
NOTE: Begin this step after the subculture has reached an OD600 of ~1.0-1.2 (step 2.3.3).
- Transfer the subculture to a 15 mL centrifuge tube, and centrifuge at 3,900 x g for 3 min at room temperature to pellet the cells. Remove the supernatant by pipetting or decanting.
- Resuspend the pellet with 1 mL of 2 mM NaPB (step 1.1), and transfer the suspension to a 1.7 mL centrifuge tube.
- Pellet the cells (as in step 4.1), discard the supernatant, and again resuspend the pellet in 1 mL of 2 mM NaPB.
- Repeat step 4.3 two additional times to leave the washed cells in 1 mL of 2 mM NaPB.
- Determine the cell density of the washed suspension, and calculate the dilution factor needed to obtain a cell density of 5 x 105 cells/mL. This concentration will ultimately lead to adding 1 x 104 cells to each well of the 96-well plate.
NOTE: The cell density of the suspension may be determined using a number of methods, including a hemocytometer or automated cell counter. A standard curve of cell density versus OD600 for the strain of interest grown under relevant conditions was used for the present study. This curve was prepared using a hemocytometer (see Table of Materials) to determine the cell density of suspensions with varying OD600 values. - Dilute the cell suspension to 5 x 105 cells/mL in 2 mM NaPB. Preparing 10 mL of this diluted suspension will be more than sufficient for completing this study.
5. Incubation of C. albicans with peptide solutions and preparation of the cells for the quantification of viability
- Add 20 µL of the diluted C. albicans suspension (step 4.6) to Columns 1-10 and Column 12 of each row (Figure 1).
NOTE: The final peptide concentration in the first column is now half of the peptide stock concentration. For the present study, the final peptide concentration was 50 µM at this point. The final cell suspension in each well contains 2.5 x 105 cells/mL. Columns 1-10 serve as experimental wells to evaluate the antifungal activity at different peptide concentrations, and Column 12 serves as a control for growth with no peptide. - Add 20 µL of 2 mM NaPB to Column 11. All the wells now have a final concentration of 1 mM NaPB. Column 11 serves as a sterility control.
- Cover Plate 1 (containing both cells and peptide), and incubate it at 30 °C for 30 min.
NOTE: An incubation time of 30 min is sufficient for many peptides17,21,22,30, but the incubation time can be increased to 60 min if desired to account for peptides that may require a longer time to exert their antifungal activity25,31,32. - Prepare a new 96-well culture plate (Plate 2) for use in quantifying the viability (Figure 2).
NOTE: The culture plate should be prepared during the incubation of Plate 1.- Add 100 µL of YPD (step 1.2) to all the wells of the plate.
- Add 100 µL of 2 mM NaPB (Step 1.1) to all the wells of the plate.
- Dilute the samples in Plate 1 (containing cells and peptides), and transfer to Plate 2 (containing medium and buffer).
- Retrieve Plate 1 from the incubator following the 30 min of incubation.
- Dilute each well of Plate 1 by adding 280 µL of 1 mM NaPB (step 1.1) for a total volume of 320 µL in each well (Figure 2).
- Mix all the wells containing cells and peptides by pipetting up and down to ensure all the cells are resuspended and no settled cells are visible at the bottom of the wells.
- Transfer 8 µL from each well in Plate 1 to the corresponding well of Plate 2. This volume transfers approximately 250 cells from each well of Plate 1 into Plate 2 (Figure 2).
- Cover Plate 2 and incubate on a microtiter plate shaker (see Table of Materials) at 350 rpm for 17 h at 30 °C.
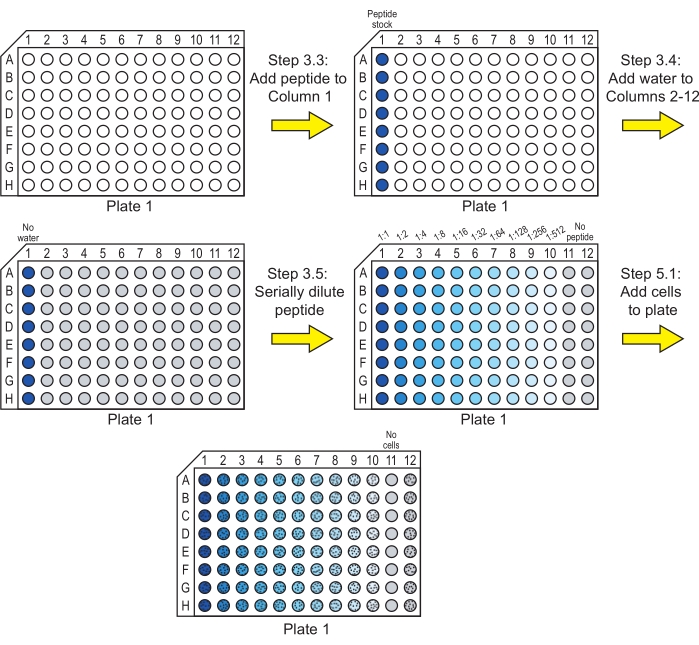
Figure 1: Preparation of Plate 1 for the incubation of peptide serial dilutions with cells. Serial dilutions of the peptide are made in water, and C. albicans cells are added to Plate 1. A blue color indicates that peptide is present in the well, and gray indicates a well with water and no peptide. Wells containing cells are indicated with a pattern of black dots. After these steps, the plate is incubated to allow the peptide to exert its antifungal activity. Please click here to view a larger version of this figure.

Figure 2: Preparation of Plate 2 for quantifying the reduction in viability due to the peptide. YPD medium and NaPB are added to Plate 2. After diluting the contents of Plate 1, an aliquot from each well is transferred from Plate 1 to Plate 2. Plate 2 is then incubated to allow any viable cells to grow for quantification by measuring the OD600. For Plate 2, wells containing only YPD and NaPB are shown in orange. Wells containing aliquots from Plate 1 mixed with the YPD and NaPB are shown in green. See the Figure 1 legend for the description of the colors on Plate 1. Please click here to view a larger version of this figure.
6. Determination of antifungal activity
- Obtain OD600 readings for each well in Plate 2.
- Remove Plate 2 from the incubator after 17 h of incubation.
- Mix all the wells in Plate 2 by pipetting up and down to ensure all the cells are resuspended and no settled cells are visible at the bottom of the wells.
NOTE: Avoid generating bubbles during this step, as they may interfere with the OD600 readings. If bubbles form, they can often be popped with a dry pipet tip or removed by using two pipet tips to lift them out of the well. - Use an absorbance plate reader (see Table of Materials) at a wavelength of 600 nm to obtain the OD600 for each well.
- Calculate the inhibition of growth (as a percentage), and plot it to determine the effect of the peptides on the growth of C. albicans.
- For each well, calculate the reduction in viability compared to the control (as a percentage) using the following equation17,22,23:
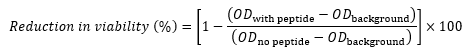
where ODwith peptide is the OD600 of a well containing a given concentration of peptide, ODbackground is the OD600 of Column 11 in the same row (control containing no cells), and
ODno peptide is the OD600 of Column 12 in the same row (control containing cells and no peptide). - For example, use the following expression to calculate the reduction in viability in Well B5 from the OD600 values for Row B:
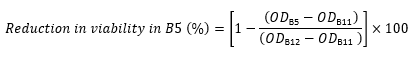
- For each well, calculate the reduction in viability compared to the control (as a percentage) using the following equation17,22,23:
- Plot the reduction in viability as a function of the peptide concentration.
Results
Using OD600 measurements to quantify the reduction in growth due to antifungal peptides saves substantial time compared to plating samples and counting CFUs. The method described in this protocol requires completing the steps on three different days. On the first day, approximately 1 h is needed to prepare the buffers and media (not including the sterilization time) and inoculate the starting culture of C. albicans for overnight incubation. On the second day, the steps require 5-6 h (including subcult...
Discussion
This protocol describes an efficient approach for obtaining quantitative data on the antifungal activity of AMPs against the fungal pathogen C. albicans. One common alternative approach to testing peptides and other antifungal agents is the broth microdilution described in the Clinical Laboratory Standards Institute's (CLSI) standard M2718, but this standard focuses on obtaining qualitative visual results instead of quantitative results. Another alternative approach is to use a method...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
This work was supported by the National Institutes of Health (R03DE029270, T32AI089621B), the National Science Foundation (CBET 1511718), the Department of Education (GAANN-P200A180093), and a University of Maryland Cross-Campus Seed Grant.
Materials
| Name | Company | Catalog Number | Comments |
| 96-well plates (round bottom) | VWR | 10062-902 | |
| Absorbance microplate reader | N/A | N/A | Any available microplate reader is sufficient |
| C. albicans strain SC5314 | ATCC | MYA-2876 | Other C. albicans may also be used |
| Hemocytometer | N/A | N/A | Can be used to make a standard curve relating cell number to OD600 |
| Microplate shaker | VWR | 2620-926 | |
| Peptide(s) | N/A | N/A | Peptides can be commercially synthesized by any reliable vendor; a purity of ≥95% and trifluoroacetic acid salt removal to hydrochloride salt are recommended |
| Reagent reservoirs for multichannel pipettors | VWR | 18900-320 | Simplifies pipetting into multiwell plates with multichannel pipettor |
| Sodium phosphate, dibasic | Fisher Scientific | BP332-500 | For making NaPB |
| Sodium phosphate, monobasic | Fisher Scientific | BP329-500 | For making NaPB |
| UV spectrophotometer | N/A | N/A | Any available UV spectrophotometer is sufficient |
| YPD medium powder | BD Life Sciences | 242820 | May also be made from yeast extract, peptone, and dextrose |
References
- Gulati, M., Nobile, C. J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes and Infection. 18 (5), 310-321 (2016).
- Arya, N. R., Rafiq, N. B. Candidiasis. StatPearls. , (2021).
- de Oliveira Santos, G. C., et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Frontiers in Microbiology. 9, 1351 (2018).
- Espinel-Ingroff, A. Mechanisms of resistance to antifungal agents: Yeasts and filamentous fungi. Revista Iberoamericana de Micología. 25 (2), 101-106 (2008).
- Wang, X., et al. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharmaceutica Sinica B. 11 (8), 2585-2604 (2021).
- Struyfs, C., Cammue, B. P. A., Thevissen, K. Membrane-interacting antifungal peptides. Frontiers in Cell and Developmental Biology. 9, 649875 (2021).
- Huan, Y., Kong, Q., Mou, H., Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Frontiers in Microbiology. 11, 582779 (2020).
- Sarkar, T., Chetia, M., Chatterjee, S. Antimicrobial peptides and proteins: From nature's reservoir to the laboratory and beyond. Frontiers in Chemistry. 9, 691532 (2021).
- Mahlapuu, M., Bjorn, C., Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Critical Reviews in Biotechnology. 40 (7), 978-992 (2020).
- Lei, J., et al. The antimicrobial peptides and their potential clinical applications. American Journal of Translational Research. 11 (7), 3919-3931 (2019).
- Mercer, D. K., O'Neil, D. A. Innate inspiration: Antifungal peptides and other immunotherapeutics from the host immune response. Frontiers in Immunology. 11, 2177 (2020).
- Bin Hafeez, A., Jiang, X., Bergen, P. J., Zhu, Y. Antimicrobial peptides: An update on classifications and databases. International Journal of Molecular Sciences. 22 (21), 11691 (2021).
- Xu, T., Levitz, S. M., Diamond, R. D., Oppenheim, F. G. Anticandidal activity of major human salivary histatins. Infection and Immunity. 59 (8), 2549-2554 (1991).
- Helmerhorst, E. J., et al. Amphotericin B- and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrobial Agents and Chemotherapy. 43 (3), 702-704 (1999).
- Andra, J., Berninghausen, O., Leippe, M. Cecropins, antibacterial peptides from insects and mammals, are potently fungicidal against Candida albicans. Medical Microbiology and Immunology. 189, 169-173 (2001).
- do Nascimento Dias, J., et al. Mechanisms of action of antimicrobial peptides ToAP2 and NDBP-5.7 against Candida albicans planktonic and biofilm cells. Scientific Reports. 10, 10327 (2020).
- Ikonomova, S. P., et al. Effects of histatin 5 modifications on antifungal activity and kinetics of proteolysis. Protein Science. 29, 480-493 (2020).
- Clinical Laboratory Standards Institute. . M27-A3. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standard - Third edition. , (2008).
- Lupetti, A., et al. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrobial Agents and Chemotherapy. 44 (12), 3257-3263 (2000).
- Han, J., Jyoti, M. A., Song, H. Y., Jang, W. S. Antifungal activity and action mechanism of histatin 5-halocidin hybrid peptides against Candida ssp. PLoS One. 11 (2), 0150196 (2016).
- den Hertog, A. L., et al. Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochemical Journal. 388, 689-695 (2005).
- Ikonomova, S. P., Moghaddam-Taaheri, P., Jabra-Rizk, M. A., Wang, Y., Karlsson, A. J. Engineering improved variants of the antifungal peptide histatin 5 with reduced susceptibility to Candida albicans secreted aspartic proteases and enhanced antimicrobial potency. The FEBS Journal. 285 (1), 146-159 (2018).
- Moghaddam-Taaheri, P., Leissa, J. A., Eppler, H. B., Jewell, C. M., Karlsson, A. J. Histatin 5 variant reduces Candida albicans biofilm viability and inhibits biofilm formation. Fungal Genetics and Biology. 149, 103529 (2021).
- Gong, Z., Doolin, M. T., Adhikari, S., Stroka, K. M., Karlsson, A. J. Role of charge and hydrophobicity in translocation of cell-penetrating peptides into Candida albicans cells. AIChE Journal. 65 (12), 16768 (2019).
- Gong, Z., Karlsson, A. J. Translocation of cell-penetrating peptides into Candida fungal pathogens. Protein Science. 26 (9), 1714-1725 (2017).
- Green, M. R., Sambrook, J. . Molecular Cloning: A Laboratory Manual. Fourth edition. 3, (2012).
- Consolidated Sterilizer Systems. Laboratory and Research Autoclaves Available from: https://consteril.com/wp-content/uploads/2020/12/CSS-Product-Brochure.pdf (2022)
- Rodriguez-Tudela, J. L., Cuenca-Estrella, M., Diaz-Guerra, T. M., Mellado, E. Standardization of antifungal susceptibility variables for a semiautomated methodology. Journal of Clinical Microbiology. 39 (7), 2513-2517 (2001).
- Mbuayama, K. R., Taute, H., Strmstedt, A. A., Bester, M. J., Gaspar, A. R. M. Antifungal activity and mode of action of synthetic peptides derived from the tick OsDef2 defensin. Journal of Peptide Science. 28 (5), 3383 (2022).
- Rossignol, T., Kelly, B., Dobson, C., d'Enfert, C. Endocytosis-mediated vacuolar accumulation of the human ApoE apolipoprotein-derived ApoEdpL-W antimicrobial peptide contributes to its antifungal activity in Candida albicans. Antimicrobial Agents and Chemotherapy. 55 (10), 4670-4681 (2011).
- Helmerhorst, E. J., Reijnders, I. M., van't Hof, W., Veerman, E. C., Nieuw Amerongen, A. V. A critical comparison of the hemolytic and fungicidal activities of cationic antimicrobial peptides. FEBS Letters. 449 (2-3), 105-110 (1999).
- Kerenga, B. K., et al. Salt-tolerant antifungal and antibacterial activities of the corn defensin ZmD32. Frontiers in Microbiology. 10, 795 (2019).
- Lee, I. H., Cho, Y., Lehrer, R. I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infection and Immunity. 65 (7), 2898-2903 (1997).
- Li, X. S., Reddy, M. S., Baev, D., Edgerton, M. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. Journal of Biological Chemistry. 278 (31), 28553-28561 (2003).
- Rothstein, D. M., et al. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrobial Agents and Chemotherapy. 45 (5), 1367-1373 (2001).
- Sanders, E. R. Aseptic laboratory techniques: Volume transfers with serological pipettes and micropipettors. Journal of Visualized Experiments. (63), e2754 (2012).
- Mansoury, M., Hamed, M., Karmustaji, R., Al Hannan, F., Safrany, S. T. The edge effect: A global problem. The trouble with culturing cells in 96-well plates. Biochemistry and Biophysics Report. 26, 100987 (2021).
- Goughenour, K. D., Balada-Llasat, J. M., Rappleye, C. A. Quantitative microplate-based growth assay for determination of antifungal susceptibility of Histoplasma capsulatum yeasts. Journal of Clinical Microbiology. 53 (10), 3286-3295 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved