A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Novel In Vivo Micro-Computed Tomography Imaging Techniques for Assessing the Progression of Non-Alcoholic Fatty Liver Disease
* These authors contributed equally
In This Article
Summary
Using a diet-induced non-alcoholic fatty liver disease (NAFLD) mouse model, we describe the use of novel in vivo micro-computed tomography imaging techniques as a non-invasive method to assess the progression stages of NAFLD, focusing predominantly on the hepatic vascular network due to its significant involvement in NAFLD-related hepatic dysregulation.
Abstract
Non-alcoholic fatty liver disease (NAFLD) is a growing global health issue, and the impact of NAFLD is compounded by the current lack of effective treatments. Considerable limiting factors hindering the timely and accurate diagnosis (including grading) and monitoring of NAFLD, as well as the development of potential therapies, are the current inadequacies in the characterization of the hepatic microenvironment structure and the scoring of the disease stage in a spatiotemporal and non-invasive manner. Using a diet-induced NAFLD mouse model, we investigated the use of in vivo micro-computed tomography (CT) imaging techniques as a non-invasive method to assess the progression stages of NAFLD, focusing predominantly on the hepatic vascular network due to its significant involvement in NAFLD-related hepatic dysregulation. This imaging methodology allows for longitudinal analysis of liver steatosis and functional tissue uptake, as well as the evaluation of the relative blood volume, portal vein diameter, and density of the vascular network. Understanding the adaptations of the hepatic vascular network during NAFLD progression and correlating this with other ways of characterizing the disease progression (steatosis, inflammation, fibrosis) using the proposed method can pave the way toward the establishment of new, more efficient, and reproducible approaches for NAFLD research in mice. This protocol is also expected to upgrade the value of preclinical animal models for investigating the development of novel therapies against disease progression.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a metabolic disease that affects approximately 25% of the population and >80% of morbidly obese people1. An estimated one-third of these individuals progress to non-alcoholic steatohepatitis (NASH), which is characterized by hepatic steatosis, inflammation, and fibrosis2. NASH is a disease stage with a significantly higher risk for the development of cirrhosis and hepatocellular carcinoma (HCC)3,4. For this reason, NASH is currently the second most common cause of liver transplantation, and it is also expected to soon become the most important predictor of liver transplantation5,6,7. Despite its prevalence and severity, no disease-specific therapy is available for NAFLD, and the existing treatments only aim to tackle disease-associated pathologies such as insulin resistance and hyperlipidemia5,6.
In recent years, the pathophysiological role and adaptations of the endothelium and, in general, of the vascular network of metabolic tissues, such as the adipose tissue and the liver, have been gaining more importance in research, especially during obesity and metabolic dysregulation7,8. The endothelium is a cellular monolayer that lines the vascular network internally, acting as a functional and structural barrier. It also contributes to various physiological and pathological processes, such as thrombosis, metabolite transport, inflammation, and angiogenesis9,10. In the case of the liver, the vascular network is, among other features, characterized by the presence of highly specialized cells, defined as liver sinusoidal endothelial cells (LSECs). These cells lack a basement membrane and have multiple fenestrae, allowing for the easier transfer of substrates between the blood and liver parenchyma. Due to their distinctive anatomical location and characteristics, LSECs likely have a crucial role in the pathophysiological processes of the liver, including the development of liver inflammation and fibrosis during NAFLD/NASH. Indeed, the pathological, molecular, and cellular adaptations that LSECs undergo in the course of NAFLD contribute to the disease progression11. Specifically, the LSEC-dependent hepatic angiogenesis that takes place during NAFLD is significantly associated with the development of inflammation and the progression of the disease to NASH or even HCC12. Besides, obesity-related early NAFLD is characterized by the development of insulin resistance in LSECs, which precedes the development of hepatic inflammation or other advanced NAFLD signs13.
Additionally, LSECs have recently emerged as central regulators of hepatic blood flow and vascular network adaptations during liver disease of several etiologies14,15. Indeed, chronic liver disease is characterized by prominent intra-hepatic vasoconstriction and increased resistance to blood flow, which contribute to the development of portal hypertension16. In the case of NAFLD, several LSEC-related mechanisms contribute to this phenomenon. For instance, LSEC-specific insulin resistance, as mentioned above, is associated with reduced insulin-dependent vasodilation of the hepatic vasculature13. Besides, over the course of the disease, the liver vasculature becomes more sensitive to vasoconstrictors, further contributing to impairment of the hepatic blood flow and leading to the emergence of shear stress, which both result in a disruption of the sinusoidal microcirculation17. These facts suggest that the vasculature is a key target in liver disease. Nevertheless, limiting factors hindering the timely diagnosis and monitoring of NAFLD/NASH, as well as the development of potential therapies, are the inadequacies in the consistent characterization of the hepatic microenvironment and (micro)vascular structure, as well as the scoring of the disease stage in a spatiotemporal and non-invasive manner.
Micro-computed tomography (CT) imaging is currently the gold-standard non-invasive imaging method for accurately depicting anatomical information within a living organism. Micro-CT and MRI represent two complementary imaging methods that can cover a vast range of pathologies and provide exceptional resolution and detail in the imaged structures and tissues. Micro-CT, in particular, is a very fast and accurate tool that is often used for studying pathologies such as bone diseases and associated bone surface changes18, assessing the progression of pulmonary fibrosis over time19, diagnosing lung cancer and its staging20, or even examining dental pathologies21, without any special preparation (or destruction) of the samples being imaged.
The imaging technology of micro-CT is based on the different attenuation properties of various organs in terms of the interaction of X-rays with matter. Organs presenting high X-ray attenuation differences are depicted with high contrast in CT images (i.e., the lungs appear dark and the bones light). Organs presenting very similar attenuation properties (different soft tissues), are challenging to distinguish on CT images22. To address this limitation, specialized contrast agents based on iodine, gold, and bismuth have been extensively investigated for in vivo use. These agents alter the attenuation properties of the tissues in which they accumulate, are cleared slowly from the circulation, and enable the uniform and stable opacification of the entire vascular system or chosen tissues23.
In human diagnostics, CT imaging and comparable techniques, such as MRI-derived proton density fat fraction, are already in use for the determination of hepatic fat content24,25. In the context of NAFLD, high soft tissue contrast is essential to accurately distinguish pathological lesions or small vessels. For this purpose, contrast agents providing enhanced contrast of the liver tissue characteristics are utilized. Such tools and materials allow for the study of multiple liver characteristics and possible pathology expressions, such as the architecture and density of the vascular network, lipid deposition/steatosis, and functional tissue uptake/lipid (chylomicron) transfer in the liver. Additionally, hepatic relative blood volume and portal vein diameter can also be evaluated. In a very short scan time, all these parameters provide different and complementary information on the evaluation and progression of NAFLD, which can be used to develop a non-invasive and detailed diagnosis.
In this article, we provide a step-by-step protocol for the use of novel in vivo micro-CT imaging techniques as a non-invasive method to assess the progression stages of NAFLD. Using this protocol, the longitudinal analysis of liver steatosis and functional tissue uptake, as well as the evaluation of the relative blood volume, portal vein diameter, and density of the vascular network, can be performed and applied in mouse models of liver disease.
Protocol
All procedures were carried out by BIOEMTECH's personnel in accordance with European and national welfare regulations and were approved by national authorities (license number EL 25 BIOexp 45/PN 49553 21/01/20). All experiments were designed and reported with adherence to ARRIVE guidelines26. The mice were purchased from the Hellenic Pasteur Institute, Athens, Greece.
NOTE: Animals were group-housed in individually ventilated cages enriched with rails and cardboard tubes in a room at 20-22 °C, with a relative humidity of 50%-60% and a 12 h light/dark cycle (light 07:00 am-07:00 pm). A combination of a high-fat diet (HFD) and high-fructose corn syrup (HFCS), a fructose- and glucose-containing sweetener widely used in modern types of fat-enriched diets, was used to induce NAFLD as a recognized reliable model27,28,29,30. At 7-8 weeks of age, male C57BL/6 mice were given ad libitum access to either a normal diet (n = 2) with 10% of kilocalories from fat or a HFD (n = 2) containing 60% of kilocalories from fat supplemented with 5% HFCS in water for 22 weeks. Body weight was obtained weekly using a digital balance, and during the experimental period, animal welfare was monitored on alternate days using a score sheet. At the end of the imaging protocol, the mice were euthanized via cervical dislocation.
1. Animal preparation
NOTE: The imaging protocol is summarized in Figure 1.
- Anesthetize the mouse using 3%-4% isoflurane (in room air), and maintain its body temperature using a dedicated heating pad.
NOTE: The absence of a pedal withdrawal reflex must be used to confirm sufficient anesthesia depth before initiating the scan. - Apply ophthalmic ointment on the animal's eyes prior to experimentation.
- Place the animal in the CT scanner cradle, secure the nose cone, and switch to 1.5%-3% isoflurane (in room air) for maintenance.
NOTE: The absence of a pedal withdrawal reflex must be used to confirm the appropriate percentage of isoflurane for the maintenance of anesthesia. - Monitor the mouse continuously.
2. Pre-scanning preparation
NOTE: Imaging is performed in two experimental phases to allow for the first contrast agent to be adequately cleared from the circulation and tissues. eXIA (first contrast agent) is administered in the first phase and ExiTron (second contrast agent) in the second phase, as described in the "Imaging workflow" section (section 3) below.
- Allow for the contrast agent (either eXIA or ExiTron, depending on the experimental phase) to reach room temperature for 3 h.
- Set the following scanning parameters on the CT scanner: high-resolution protocol under 50 kVp tube voltage and a current of 460 µA, non-spiral, 720 projections/rotation, four rotations, and 4 min acquisition time.
3. Imaging workflow
- Experimental phase 1
- Calculate and prepare the volume of the first contrast agent to be administered at an undiluted dose of 6 µL/g of body weight for maximum contrast.
- Prepare the tail vein catheter by filling it up with saline and connecting it to the syringe filled with the contrast agent.
- Acquire a pre-contrast whole body (WB) and liver baseline scan.
- Ensure that there are no bubbles or blockages in the syringe or catheter.
- Insert the prefilled catheter into the tail vein, and administer the contrast agent via an injection performed slowly and manually, with a duration of 1-3 min (not as a bolus injection). A syringe pump can be used when set to the appropriate infusion rate.
NOTE: The tail of the animal can be placed in lukewarm water to induce vasodilation and help with the catheter insertion - Acquire WB and liver scans at different time points, as indicated in Table 1.
NOTE: If the acquisition of all points is not possible, the focus should be placed on 45 min post-injection (PI), which is the point of maximum liver uptake, and 48 h PI, which is when clearance is achieved.
- Experimental phase 2
- Prepare the mouse again as described in section 1 for the administration of the second contrast agent 10 days following the final reading with the first contrast agent (48 h PI).
- Perform steps 2.1-2.2.
- Calculate and prepare the volume of the second contrast agent to be administered at an undiluted dose of 8 µL/g of body weight for maximum contrast.
- Prepare the tail vein catheter by filling it up with saline and connecting it to the syringe filled with the contrast agent.
- Acquire a pre-contrast WB and liver baseline scan to evaluate the relative blood volume and liver steatosis.
- Ensure no contrast is detectable in the scan as an indication of the complete clearance of the first contrast agent.
- Insert the prefilled catheter into the tail vein, and administer the contrast agent via an intravenous injection performed slowly and manually, with a duration of 1-3 min (not as a bolus injection). A syringe pump can be used when set to the appropriate infusion rate.
- Acquire WB and liver scans at different time points, as indicated in Table 1.
NOTE: WB scans are acquired at 10 min and 4 h PI. The significant time lapse between them allows for the evaluation of the tracer biodistribution in the body as well as its relative clearance.
4. Data extraction and analysis
NOTE: In this protocol, the data extraction and analysis steps based on a specific imaging processing software (see Table of Materials) are provided. The described steps may need to be adapted when using different software.
- Evaluation of hepatic lipid deposition/steatosis.
NOTE: For the evaluation of hepatic steatosis, no contrast agent is used, and a comparison between control and pathology is performed. Due to relatively high deviations in tissue attenuation properties between different mice, the density values are normalized for the liver against the spleen (fat-free tissue) and the fat (absolute fat tissues) according to the following equation and as previously described25:
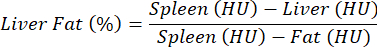
- To perform the analysis, load the DICOM file of the pre-contrast scan, and adjust the bar/contrast to see the liver, spleen, and white adipose tissue (WAT) clearly.
- Access the Modelling Operator tool via the tool pull-down menu on the front panel, and select 3D ROI Tool.
- Under the 3D ROI Operator, select Add ROI to generate multiple ROIs (up to eight for each tissue) to perform sampling in the areas where the liver (preferably in the areas of the left medial lobe, the right medial lobe, and the left lateral lobe) and spleen appear clear, with no apparent blood vessels and fat.
NOTE: For WAT, ROIs are selected in the middle of the visceral adipose tissue depot. Recommended areas are shown in Figure 2. Normalization methods using the liver/spleen ratio and without including WAT can also be applied as previously established31. - Under the 3D Paint Mode and Erode/Dilate feature, select 2D, and use the interface that appears to specify a name and color for each ROI.
- Use the Sphere paint ROI tool with a diameter of 8 pixels to manually draw the 2D ROIs.
- Perform sampling by segmenting the 2D ROIs on the areas of interest using the Cross Hair tool on the transverse plane, as shown in Figure 3A.
- Click on the selected point at the sagittal and coronal plane to complete the segmentation of the 2D ROI, as shown in Figure 3B.
- Repeat the process for defining the rest of the ROIs.
NOTE: When sampling, avoid the organ border regions, as this can introduce noise and affect the reliability of the calculated Hounsfield unit (HU) value of each ROI. - Once satisfied with the segmented ROIs, go to Navigation, and select Show Table to display the quantification table containing the calculated HU values for each ROI.
NOTE: The values of interest are listed in the "Mean" column, which contains the numerical mean values of the voxels (HU) contained in the ROIs for the organs of interest. Note the values of interest, or save the entire table by selecting Export Table. - Calculate the average HU for the liver, spleen, and WAT, and plug the values into the above equation to calculate the percentage of liver fat.
- Functional tissue uptake/lipid (chylomicron) transfer in the liver
NOTE: Functional tissue uptake/lipid (chylomicron) transfer is analyzed from the acquired scans at 45 min and 48 h following the first contrast agent infusion, based on a previously published method32. The contrast is calculated for the different tissues and time points using the equation below:
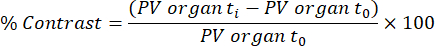
PV organ ti is the average pixel value in the organ at time ti (ranging from 0 h to 48 h), and PV organ t0 is the average pixel value in the organ in the image without contrast.- To perform this analysis, load the eXIA scan DICOM file, and adjust the bar/contrast to see the liver, spleen, and left ventricle clearly.
- Access the Modelling Operator via the tool pull-down menu on the front panel, and select 3D ROI Tool.
- Under the 3D ROI Operator, select Add ROI to segment multiple ROIs for the liver.
- Under the 3D Paint Mode and Erode/Dilate feature, use the Sphere paint ROI tool with a diameter of 8 pixels and −1 erode.
NOTE: Select multiple ROIs on slices where each organ appears clearly. Avoid border regions, as this can introduce noise and affect the reliability of the calculated HU value of each ROI. This will result in the sampling of multiple 3D ROIs, which correspond to small organ volumes. - Use the interface that appears to specify a name and color for each ROI.
- Once satisfied with the designed ROIs, go to Navigation, and select Show Table to display the quantification table containing the calculated HU values for each ROI.
NOTE: The values of interest are listed under the "Mean" column, which displays the numerical mean values of the voxels (HU) included in the ROI. The average HU value of each organ's ROIs corresponds to PV organ ti. Note the values of interest, or save the entire table by selecting Export Table. - To obtain PV organ t0, repeat all of the above steps using the pre-contrast DICOM file to calculate the mean brightness of the liver, spleen, and left ventricle before the contrast agent injection.
- Insert the values into the above equation to extract the percentage contrast corresponding to the functional tissue uptake/lipid (chylomicron) transfer.
- Architecture and density of the hepatic vascular network
NOTE: The analysis of the architecture and density of the hepatic vascular network is based on a previously published methodology33 and is performed on the liver scans obtained 10 min PI of the second contrast agent.- To perform this analysis, load the ExiTron scan DICOM file, and adjust the bar/contrast to see the liver vascular network clearly.
- Access the Modelling Operator via the tool pull-down menu on the front panel, and select 3D ROI Tool.
- Under the 3D ROI Operator, select Add ROI to generate a 3D ROI for the liver.
- Under the 3D Paint Mode and Erode/Dilate feature, select 3D.
NOTE: Use the Sphere paint ROI tool with −1 erode to define the segmentation layers across the coronal plane. The diameter of the ROI painting tool must be adjusted according to each layer (for adding/deleting any wanted/unwanted voxel selections). It is recommended that the liver volume be initially defined across the coronal plane, and then the transverse and sagittal planes can be used to correct the ROI. This process requires precision. The user must be very careful not to include other tissues, vessels, and bones when segmenting each ROI layer while ensuring that all areas of the liver are included in the defined ROI. For this reason, familiarization with the anatomical boundaries of the liver is crucial. - Once satisfied with the resulting liver ROI, perform a cut in order to remove all the voxels from the image data that do not belong in the initially segmented liver ROI. For this, choose the liver ROI from the ROI Selector, and click on the Perform Cut icon. This operation removes the background and leaves the liver ROI unchanged.
NOTE: Although the undo/redo functions are applicable to all the operations performed under the 3D ROI Tool, the action of cutting an ROI cannot be undone. So, prior to this action, the user might consider saving the initial liver ROI in DICOM format. - The resulting liver ROI includes the vascular network and the surrounding tissue, which must be removed. For this, reset the liver ROI by clicking on the Reset ROI broom button.
- Use the interface that appears to transfer all the pixels of the liver ROI to the background.
NOTE: The liver ROI will still exist after this operation, but it will no longer contain any voxels. - To re-segment the liver ROI so that it only contains vascular-associated pixels, go to Segmentation Algorithms denoted by the magic wand icon, and select Connected Thresholding.
- Define the ROI as Output and the background as Input from the input drop-down menu before applying thresholding.
- Set the Thresholds by clicking the Min and Max icons to the left of each threshold field to fill in maximum and minimum values and obtain the vascular network.
NOTE: Only pixels within the chosen range will be included in the resulting ROI. Adjusting the threshold values between different animals ensures that the same anatomical regions are taken into account with respect to the exact amount of contrast agent injected into each animal. This is constant among the selected tissues even if the numerical values are not identical. - Use the Cross Hair tool to click on a point where the vascular network appears clear, and click on Apply to perform the segmentation.
- Activate the maximum intensity projection (MIP) viewer.
- Evaluate the resulting liver ROI in terms of how clear the vascular network appears in the MIP view.
- If the tissue remains in parts of the liver ROI, repeat steps 4.3.5-4.3.11 by adjusting the Min threshold value until the segmented liver ROI clearly represents the vascular network.
- Once satisfied with the resulting liver ROI, generate the quantification table containing the calculated liver ROI volume in cubic millimeters.
NOTE: The values of interest are listed in the "mm3" column, which contains the numerical volume value of the voxels (HU) contained in the liver ROI. Note the values of interest, or save the entire table by selecting Export Table.
- Hepatic relative blood volume
NOTE: For the measurement of the hepatic relative blood volume (rBV), which correlates significantly with the amount of newly formed blood vessels during fibrosis progression, pre-contrast scans and scans at 4 h after the second contrast agent injection are used. The analysis is performed as previously described34.- To perform this analysis, load the ExiTron scan DICOM file, and adjust the bar/contrast.
NOTE: Disable the MIP viewer: under Preferences, check the box to disable the MIP viewer upon loading. For large datasets, this can improve the loading speed. - Access the Modelling Operator via the tool pull-down menu on the front panel, and select 3D ROI Tool. This tool provides advanced options for drawing, visualizing, saving, and quantifying both 2D and 3D regions.
- Under the 3D ROI Operator, select Add ROI, and segment two ROIs: one for the liver and one for a large blood vessel.
- Under the 3D Paint Mode and Erode/Dilate feature, select 2D.
NOTE: It is recommended to use the Sphere paint ROI tool with a diameter of 8-10 pixels for the liver and 4-6 pixels for the blood vessel. However, the paint tool diameter can be adjusted depending on how small the area to be selected is. - Use the interface that appears to specify a name and color for each ROI.
NOTE: Select two to five slices of central parts of the tissues of interest, and define the segmentation layers to generate 2D ROIs for each tissue. When selecting the areas on each slice, avoid the organ border regions, as shown in Figure 4, as this can introduce noise and affect the reliability of the calculated HU value of each ROI. - Once satisfied with the designed ROIs, go to Navigation, and select Show Table to display the quantification table containing the calculated HU values for each ROI.
NOTE: The values of interest are listed under the "Mean" column, which displays the numerical mean values of the voxels (HU) included in the ROI. Note the values of interest, or save the entire table by selecting Export Table. - Repeat all the steps for the pre-contrast DICOM file to obtain the mean brightness of the liver before the contrast agent injection. For this, carry out steps 4.4.2-4.4.5 for the liver only.
- Calculate the average HU values for each tissue at the equivalent time points, and insert the values obtained into the equation below:
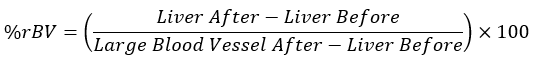
NOTE: A large blood vessel after contrast agent injection is considered 100% rBV, and the liver, before contrast agent administration, is considered 0% rBV.
- To perform this analysis, load the ExiTron scan DICOM file, and adjust the bar/contrast.
- Portal vein diameter
NOTE: For the portal vein diameter measurements, the same scans used for the hepatic rBV measurements are analyzed as previously described35.- Load the ExiTron scan DICOM file, and adjust the bar/contrast.
- Locate the transversal planes of three to four slices above the junction of the superior mesenteric and splenic veins (Figure 5).
- Use the Ruler tool to measure the exact distance between two points (i.e., the diameter of the circular vein region).
NOTE: The distance is extracted on the image, but one can also go to Navigation and select Show Table to display the quantification table containing the calculated distance or select Export Table to save the result.
Results
In this representative study, micro-CT imaging without any contrast agent indicated a higher percentage of liver fat in mice with NAFLD compared to controls (Table 2), confirming the pathology. Using the ExiTron contrast agent and the hepatic vascular network architecture and density analysis described above, the total volume density of the hepatic vascular network was found to be higher in mice with NAFLD compared to healthy controls (Figure 6, Table 2). Mi...
Discussion
The current recommended method for NAFLD diagnosis and staging in humans is liver biopsy, which harbors the risk of bleeding complexities, as well as sampling inaccuracies40. On the contrary, in animal models, such diagnosis is performed by histology post-mortem, although protocols for survivable liver biopsy are now available and are recommended when the study design allows41. The use of post-mortem histology means that a large number of animals are required to investigate...
Disclosures
The authors have nothing to disclose.
Acknowledgements
Figure 1 was created with BioRender.com. This work was supported by the Hellenic Foundation for Research and Innovation (#3222 to A.C.). Anna Hadjihambi is funded by The Roger Williams Institute of Hepatology, Foundation for Liver Research.
Materials
| Name | Company | Catalog Number | Comments |
| eXIA160 | Binitio Biomedical, Inc. | https://www.binitio.com/?Page=Products | |
| High fat diet with 60% of kilocalories from fat | Research Diets, New Brunswick, NJ, USA | D12492 | |
| High-fructose corn syrup | Best flavors, CA | hfcs-1gallon | |
| Lacrinorm ophthalmic ointment | Bausch & Lomb | ||
| Normal diet with 10% of kilocalories from fat | Research Diets, New Brunswick, NJ, USA | D12450 | |
| Viscover ExiTron nano 12000 | Milteny Biotec, Bergisch Gladbach, Germany | 130-095-698 | |
| VivoQuant | Invicro | ||
| X-CUBE | Molecubes, Belgium | https://www.molecubes.com/systems/ |
References
- Lazarus, J. V., et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nature Reviews. Gastroenterology & Hepatology. 19 (1), 60-78 (2022).
- Takahashi, Y., Fukusato, T. Histopathology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. World Journal of Gastroenterology. 20 (42), 15539-15548 (2014).
- Huang, D. Q., El-Serag, H. B., Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nature Reviews Gastroenterology & Hepatology. 18 (4), 223-238 (2021).
- Niederseer, D., Wernly, B., Aigner, E., Stickel, F., Datz, C. NAFLD and cardiovascular diseases: Epidemiological, mechanistic and therapeutic considerations. Journal of Clinical Medicine. 10 (3), 467 (2021).
- Lefere, S., et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. Journal of Hepatology. 73 (4), 757-770 (2020).
- Chrysavgis, L., Papatheodoridi, A. M., Chatzigeorgiou, A., Cholongitas, E. The impact of sodium glucose co-transporter 2 inhibitors on non-alcoholic fatty liver disease.Journal of Gastroenterology and Hepatology. Journal of Gastroenterology and Hepatology. 36 (4), 893-909 (2021).
- Li, M., Qian, M., Xu, J. Vascular endothelial regulation of obesity-associated insulin resistance. Frontiers in Cardiovascular Medicine. 4, 51 (2017).
- Pi, X., Xie, L., Patterson, C. Emerging roles of vascular endothelium in metabolic homeostasis. Circulation Research. 123 (4), 477-494 (2018).
- Chiu, J. J., Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiological Reviews. 91 (1), 327-387 (2011).
- Koyama, Y., Brenner, D. A. Liver inflammation and fibrosis. The Journal of Clinical Investigation. 127 (1), 55-64 (2017).
- Nasiri-Ansari, N., et al. Endothelial cell dysfunction and non-alcoholic fatty liver disease (NAFLD): A concise review. Cells. 11 (16), 2511 (2022).
- Lefere, S., et al. Angiopoietin-2 promotes pathological angiogenesis and is a therapeutic target in murine non-alcoholic fatty liver disease. Hepatology. 69 (3), 1087-1104 (2019).
- Pasarin, M., et al. Insulin resistance and liver microcirculation in a rat model of early NAFLD. Journal of Hepatology. 55 (5), 1095-1102 (2011).
- Hammoutene, A., Rautou, P. E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. Journal of Hepatology. 70 (6), 1278-1291 (2019).
- Sun, X., Harris, E. N. New aspects of hepatic endothelial cells in physiology and non-alcoholic fatty liver disease. American Journal of Physiology. Cell Physiology. 318 (6), C1200-C1213 (2020).
- Iwakiri, Y., Shah, V., Rockey, D. C. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. Journal of Hepatology. 61 (4), 912-924 (2014).
- Baffy, G. Origins of portal hypertension in non-alcoholic fatty liver disease. Digestive Diseases and Sciences. 63 (3), 563-576 (2018).
- Ruhli, F. J., Kuhn, G., Evison, R., Muller, R., Schultz, M. Diagnostic value of micro-CT in comparison with histology in the qualitative assessment of historical human skull bone pathologies. American Journal of Physical Anthropology. 133 (4), 1099-1111 (2007).
- Rodt, T., et al. Micro-computed tomography of pulmonary fibrosis in mice induced by adenoviral gene transfer of biologically active transforming growth factor-beta1. Respiratory Research. 11 (1), 181 (2010).
- Deng, L., Xiao, S. M., Qiang, J. W., Li, Y. A., Zhang, Y. Early lung adenocarcinoma in mice: Micro-computed tomography manifestations and correlation with pathology. Translational Oncology. 10 (3), 311-317 (2017).
- Feng, J., et al. Abnormalities in the enamel in bmp2-deficient mice. Cells, Tissues, Organs. 194 (2-4), 216-221 (2011).
- Kagadis, G. C., Loudos, G., Katsanos, K., Langer, S. G., Nikiforidis, G. C. In vivo small animal imaging: current status and future prospects. Medical Physics. 37 (12), 6421-6442 (2010).
- Starosolski, Z., et al. Ultra high-resolution in vivo computed tomography imaging of mouse cerebrovasculature using a long circulating blood pool contrast agent. Scientific Reports. 5, 10178 (2015).
- Caussy, C., Reeder, S. B., Sirlin, C. B., Noninvasive Loomba, R. quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 68 (2), 763-772 (2018).
- Lubura, M., et al. Non-invasive quantification of white and brown adipose tissues and liver fat content by computed tomography in mice. PLoS One. 7 (5), e37026 (2012).
- Perciedu Sert, N., et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology. 18 (7), e3000410 (2020).
- Tetri, L. H., Basaranoglu, M., Brunt, E. M., Yerian, L. M., Neuschwander-Tetri, B. A. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. American Journal of Physiology. Gastrointestinal and Liver Physiology. 295 (5), G987-G995 (2008).
- Machado, M. V., et al. Mouse models of diet-induced non-alcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 10 (5), 0127991 (2015).
- Jensen, T., et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. Journal of Hepatology. 68 (5), 1063-1075 (2018).
- Nevzorova, Y. A., Boyer-Diaz, Z., Cubero, F. J., Gracia-Sancho, J. Animal models for liver disease - A practical approach for translational research. Journal of Hepatology. 73 (2), 423-440 (2020).
- De Rudder, M., et al. Automated computerized image analysis for the user-independent evaluation of disease severity in preclinical models of NAFLD/NASH. Laboratory Investigation. 100 (1), 147-160 (2020).
- Willekens, I., et al. Time-course of contrast enhancement in spleen and liver with Exia 160, Fenestra LC, and VC. Molecular Imaging and Biology. 11 (2), 128-135 (2009).
- Das, N. M., et al. In vivo quantitative microcomputed tomographic analysis of vasculature and organs in a normal and diseased mouse model. PLoS One. 11 (2), e0150085 (2016).
- Ehling, J., et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 63 (12), 1960-1971 (2014).
- Zhang, J., et al. Gamna-Gandy bodies of the spleen detected with susceptibility weighted imaging: maybe a new potential non-invasive marker of esophageal varices. PLoS One. 8 (1), e55626 (2013).
- Chen, Y., Li, J., Zhou, Q., Lyu, G., Li, S. Detection of liver and spleen stiffness in rats with portal hypertension by two-dimensional shear wave elastography. BMC Medical Imaging. 22 (1), 68 (2022).
- Lessa, A. S., et al. Ultrasound imaging in an experimental model of fatty liver disease and cirrhosis in rats. BMC Veterinary Research. 6, 6 (2010).
- Abikhzer, G., Alabed, Y. Z., Azoulay, L., Assayag, J., Rush, C. Altered hepatic metabolic activity in patients with hepatic steatosis on FDG PET/CT. AJR. American Journal of Roentgenology. 196 (1), 176-180 (2011).
- Newman, E. M., Rowland, A. A physiologically based pharmacokinetic model to predict the impact of metabolic changes associated with metabolic associated fatty liver disease on drug exposure. International Journal of Molecular Sciences. 23 (19), 11751 (2022).
- Tsai, E., Lee, T. P. Diagnosis and evaluation of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis, including noninvasive biomarkers and transient elastography. Clinics in Liver Disease. 22 (1), 73-92 (2018).
- Oldham, S., Rivera, C., Boland, M. L., Trevaskis, J. L. Incorporation of a survivable liver biopsy procedure in mice to assess non-alcoholic steatohepatitis (NASH) resolution. Journal of Visualized Experiments. 146, e59130 (2019).
- Boll, H., et al. Comparison of Fenestra LC, ExiTron nano 6000, and ExiTron nano 12000 for micro-CT imaging of liver and spleen in mice. Academic Radiology. 20 (9), 1137-1143 (2013).
- Ashton, J. R., West, J. L., Badea, C. T. In vivo small animal micro-CT using nanoparticle contrast agents. Frontiers in Pharmacology. 6, 256 (2015).
- Rothe, J. H., et al. Time course of contrast enhancement by micro-CT with dedicated contrast agents in normal mice and mice with hepatocellular carcinoma: Comparison of one iodinated and two nanoparticle-based agents. Academic Radiology. 22 (2), 169-178 (2015).
- Toczek, J., et al. Computed tomography imaging of macrophage phagocytic activity in abdominal aortic aneurysm. Theranostics. 11 (12), 5876-5888 (2021).
- Mannheim, J. G., et al. Comparison of small animal CT contrast agents. Contrast Media & Molecular Imaging. 11 (4), 272-284 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved