A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A 3D Culture Method of Spheroids of Embryonic and Liver Zebrafish Cell Lines
In This Article
Summary
Here, we present an effective, easy, and fast 3D culture protocol for the formation of spheroids of two zebrafish (Danio rerio) cell lines: ZEM2S (embryo) and ZFL (normal hepatocyte).
Abstract
Fish cell lines are promising in vitro models for ecotoxicity assessment; however, conventional monolayer culture systems (2D culture) have well-known limitations (e.g., culture longevity and maintenance of some in vivo cellular functions). Thus, 3D cultures, such as spheroids, have been proposed, since these models can reproduce tissue-like structures, better recapturing the in vivo conditions. This article describes an effective, easy, and fast 3D culture protocol for the formation of spheroids with two zebrafish (Danio rerio) cell lines: ZEM2S (embryo) and ZFL (normal hepatocyte). The protocol consists of plating the cells in a round-bottom, ultra-low attachment, 96-well plate. After 5 days under orbital shaking (70 rpm), a single spheroid per well is formed. The formed spheroids present stable size and shape, and this method avoids the formation of multiple spheroids in a well; thus, it is not necessary to handpick spheroids of similar sizes. The ease, speed, and reproducibility of this spheroid method make it useful for high-throughput in vitro tests.
Introduction
Spheroids are small spheres of cells formed when cells are cultured in close cell-to-cell contact in 3D culture. The capacity of spheroids to mimic the in vivo tissue environment has already been studied in a variety of cell lines and primary cells1,2. However, although spheroids are well developed for mammalian toxicity studies, the development of spheroids for toxicity studies with non-mammalian vertebrates (e.g., fish) is still in progress3. For fish cell lines, spheroids have been developed by a variety of different methods, such as orbital shaking (OS) using different types of well-plates3,4,5,6,7, and the method of magnetic levitation using magnetic nanoparticles8. However, some of these culture methods for spheroids may have more disadvantages than others.
For example, gyratory methods in large microplates (24-well plates) may generate a high number of spheroids differing in size and shape; indeed, multi-spheroid structure formation has been demonstrated7. This requires intense efforts to handpick spheroids with a similar size and shape for an experiment. The hanging drop 3D culture method is commonly used for generating spheroids of mammalian cell lines1,2,9,10,11, whereby single spheroids per drop can be generated, avoiding the problems described above. However, although a modified hanging drop method (hanging drop + orbital shaking) is able to generate ZFL spheroids using an inexpensive method, it has its disadvantages12. The cellular aggregates formed cannot be maintained for long periods in the drops; thus, they need to be transferred to well plates. This process requires intense handling and long periods of work in a laminar flow hood, since it is performed dropwise using a micropipette12. In addition, this method requires 10 days to fully form the ZFL spheroids (5 days in hanging drop + 5 days in OS)12. These disadvantages can limit the application of 3D fish spheroids for toxicity testing, especially considering potential applications for chemical prioritization and product sustainability.
Thus, this article describes a 3D culture protocol able to generate single spheroids of ZFL (D. rerio normal hepatocyte) and ZEM2S (D. rerio blastula phase embryo) cell lines based on the combined use of 96-well, ultra-low attachment plates (ULA-plates) and an orbital shaker (22 mm rotational diameter). The method applied is simple and reproducible, and can generate high numbers of spheroids of similar size and shape in a short period (5 days). The advantages of this method can support the application of fish 3D models for aquatic toxicity studies in both industry and academia, as well as the progress of implementing alternative methods for ecotoxicity testing.
Protocol
The key steps to generate 3D spheroids of ZFL and ZEM2S cell lines in a round-bottom 96-well plate are presented in Figure 1.
NOTE: See the Table of Materials for details related to all materials used in this protocol and Table 1 for solutions and culture media used in this protocol.
1. Cell culture medium and monolayer cultures
- Grow both cell lines (ZFL, ZEM2S) as monolayers in an incubator at 28 °C without CO2, and subculture them at a subcultivation ratio of 1:3 when they reach ~80% confluence.
- Start with a T75 flask of zebrafish cells at ~80% confluence, cultured as described above.
- Remove the complete medium and wash the cells by adding 1x phosphate-buffered saline (PBS) (0.01 M) to the culture flask with the aid of a pipette.
- With the aid of a pipette, add 3 mL of 1x trypsin-0.5 mM EDTA (0.05% [v/v]) to the culture flasks, and incubate at 28 °C for 3 min for the detachment of cells from the flask.
- Gently tap the flask to release the cells, and then stop the trypsin digestion by adding 3 mL of complete culture medium to the flask.
- Using a pipette, transfer the cell suspension to a 15 mL conical centrifuge tube, and centrifuge at 100 × g for 5 min.
- After the pellet formation, carefully remove the supernatant, add 1 mL of the complete medium for the respective cell line in use (ZFL or ZEM2S), and resuspend using a micropipette. Take an aliquot for cell counting.
2. Cell counting with trypan blue dye exclusion test
- Add 10 µL of the cell suspension and 10 µL of trypan blue dye to a microtube to count the cells and evaluate their viability. Mix the cell suspension and dye using a pipette.
- Then, transfer 10 µL of this mixture (cell suspension + trypan blue) to a Neubauer chamber and count the cells in the four large squares (quadrants: Q) placed at the corners of the chamber, considering cells that do not take up trypan blue as viable. Calculate the number of viable cells using equation (1):
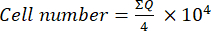 (1)
(1) - To calculate the final cell number in the cell suspension, multiply the cell number determined using equation (1) by 2 (the dilution factor due to the use of trypan blue).
NOTE: Alternatively, an automated cell counting system can be used.
3. Cell plating in ULA-plates
- After calculating the cell number, adjust the cell suspension to plate 200 µL of this suspension per well of a 96-well round-bottom ULA-plate with the number of cells required for each cell line, as indicated below:
- Plate 7,000 viable ZFL cells/well; thus, for the entire ULA-plate, use 700,000 cells in 20 mL of complete medium.
- Plate 3,500 viable ZEM2S cells/well; thus, for the entire ULA-plate, use 350,000 cells in 20 mL of complete medium.
- Prepare the cell suspension with the adjusted concentration of cells in a medium reservoir and mix it using a multichannel micropipette, taking care not to form foam or bubbles. Using the multichannel micropipette, add 200 µL of the adjusted cell suspension to each well of the ULA-plate.
NOTE: The plate must be sealed with parafilm or adhesive sealing foil to avoid culture medium evaporation from the 96-well plate.
4. Spheroid formation
- Incubate the ULA-plate on an orbital shaker at 70 rpm for 5 days in a 28 °C incubator. Allow the spheroids to form over 5 days of orbital shaking (Figure 2), reaching an average size of ~225 µm in diameter (ZFL spheroids) and ~226 µm in diameter (ZEM2S spheroids)12.
NOTE: After 5 days of incubation (maximum circularity), the spheroids are ready to be used. - To maintain the spheroids in culture for more than 5 days, remove 100 µL of the spent medium every 5 days, and add 100 µL of fresh complete culture medium using a multichannel micropipette.
NOTE: Take care not to aspirate the spheroids during this process.
5. Measuring size (diameter) and shape (circularity index) of spheroids
- Acquire the images.
- Under an inverted light microscope with an imaging capture system, obtain an image of a defined scale.
NOTE: Use a microscope stage calibration slide or a Neubauer slide (in which the quadrant sizes are known) to obtain the scale. - Under the microscope and using the same objective lens used to obtain the scale's picture, obtain images of the fully formed spheroids (i.e., 5-day-old spheroids).
NOTE: All images must be taken using the same imaging capture system, as image resolution is important to determine the spheroids' size and shape, and it may differ among types of systems.
- Under an inverted light microscope with an imaging capture system, obtain an image of a defined scale.
- Set the scale.
- Using ImageJ software, open the image of the defined scale (click File | open).
- Select the straight-line selector from the toolbar and, using the mouse, drag out a line across the extension of the defined scale in the image.
- Set the scale by selecting Analyze | Set scale, and wait for the Set Scale window to open.
- In the Set Scale window, fill the blank of Known distance with the known distance (µm) corresponding to the straight line; fill the Unit of length with um for µm. Click OK.
NOTE: The scale in pixels/µm is displayed at the bottom of the window.
- Set the measurement parameters.
- In the ImageJ software, select Analyze | Set measurements to open the Set measurements window.
- In the Set measurements window, select the boxes for the desired measurements (i.e., Area and Shape descriptors). Click OK.
- Obtain the spheroids' diameter and circularity.
- Open the picture of a spheroid (File | Open).
- Select the freehand selection tool in the toolbar and, using the mouse, select the outer side of the spheroid, as demonstrated in Figure 3A.
NOTE: To zoom in or out on the image, press the Ctrl key and use the mouse to scroll down or up, or press the Ctrl key and use the up or down arrow keys on the keyboard. - Select Analyze | Measure to open the Results window, in which the measured values are displayed.
- Using the Area value, calculate the spheroids' size (diameter) using equation (2):
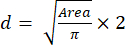 (2)
(2) - The circularity index is given in the Results window as "Circ.", and is automatically calculated by the software using equation (3):
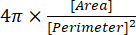 (3)
(3)
NOTE: The circularity index of 1.0 represents a perfect spheroidal shape, while a value close to 0.0 indicates an elongated shape13.
Results
Single spheroids per well with a stable size and shape are formed by this method. Figure 2 illustrates the formation process of single spheroids of ZFL and ZEM2S cells in a well of a ULA-plate under orbital shaking (70 rpm). The ZFL and ZEM2S cell lines have different behaviors in 3D culture. The ZEM2S cell line presents features that confer the ability to readily form a spheroidal shape since the first day of the orbital shaking (Figure 2E), while the ZFL cell ...
Discussion
This is a simple, easy, and fast method for generating spheroids of zebrafish liver and embryo cell lines. This method was developed by this group based on modifications of existing 3D spheroid methods to overcome problems reported in scientific studies related to spheroid formation, as well as uncertainties in data accuracy from 3D spheroid assays. For instance, the problems reported lie in difficulties of handling, the time-consuming nature of generating spheroids, the necessity of selecting spheroids of a similar size...
Disclosures
The authors declare no conflict of interest.
Acknowledgements
In memory of Dr. Márcio Lorencini, a coauthor of this work, an excellent researcher in the field of cosmetics and devoted to promoting cosmetic research in Brazil. The authors are grateful to the Multi-user Laboratory in the Physiology Department (UFPR) for equipment availability and for the financial support of the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) (Finance Code 001) and the Grupo Boticário.
Materials
| Name | Company | Catalog Number | Comments |
| 96-well Clear Round Bottom Ultra-Low Attachment Microplate, Individually Wrapped, with Lid, Sterile | Corning | 7007 | |
| DMEM, powder, high glucose, pyruvate | Gibco | 12800-017 | |
| Ham's F-12 Nutrient Mix, powder | Gibco | 21700026 | |
| HEPES (1M) | Gibco | 15630080 | |
| Image Processing and analysis in Java (ImageJ) 1.52p software | National Institutes of Health, USA | Available at: https://imagej.nih.gov/ij/index.html | |
| Leibovitz's L-15 Medium, powder | Gibco | 41300021 | |
| Orbital shaker | Warmnest | KLD-350-BI | 22 mm rotation diameter |
| Dulbeccos PBS (10x) with calcium and magnesium | Invitrogen | 14080055 | |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco | 15140122 | |
| RPMI 1640 Medium | Gibco | 31800-014 | |
| FBS - Fetal Bovine Serum, qualified, USDA-approved regions | Gibco | 12657-029 | |
| Sodium bicarbonate, powder, bioreagent for molecular biology | Sigma-Aldrich | S5761 | |
| Trypan blue stain (0,4%) | Gibco | 15250-061 | |
| Trypsin-EDTA (0.5%), no phenol red | Gibco | 15400054 | |
| ZEM2S cell line | ATCC | CRL-2147 | This cell line was kindly donated by Professor Dr. Michael J. Carvan (University of Wisconsin, Milwaukee, USA) |
| ZFL cell line | BCRJ | 256 |
References
- Elje, E., et al. The comet assay applied to HepG2 liver spheroids. Mutation Research. Genetic Toxicology and Environmental Mutagenesis. 845, 403033 (2019).
- Kelm, J. M., Timmins, N. E., Brown, C. J., Fussenegger, M., Nielsen, N. K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnology and Bioengineering. 83 (2), 173-180 (2003).
- Baron, M. G., Purcell, W. M., Jackson, S. K., Owen, S. F., Jha, A. N. Towards a more representative in vitro method for fish ecotoxicology: morphological and biochemical characterisation of three-dimensional spheroidal hepatocytes. Ecotoxicology. 21 (8), 2419-2429 (2012).
- Alves, R. F., Rocha, E., Madureira, T. V. Fish hepatocyte spheroids - A powerful (though underexplored) alternative in vitro model to study hepatotoxicity. Comparative Biochemistry and Physiology. Toxicology & Pharmacology. 262, 109470 (2022).
- Baron, M. G., et al. Pharmaceutical metabolism in fish: using a 3-D hepatic in vitro model to assess clearance. PloS One. 12 (1), 0168837 (2017).
- Langan, L. M., et al. Spheroid size does not impact metabolism of the β-blocker propranolol in 3D intestinal fish model. Frontiers in Pharmacology. 9, 947 (2018).
- Lammel, T., Tsoukatou, G., Jellinek, J., Sturve, J. Development of three-dimensional (3D) spheroid cultures of the continuous rainbow trout liver cell line RTL-W1. Ecotoxicology and Environmental Safety. 167, 250-258 (2019).
- Jeong, Y., et al. Differential effects of CBZ-induced catalysis and cytochrome gene expression in three dimensional zebrafish liver cellculture. Journal of Environmental and Analytical Toxicology. 6, 2161 (2016).
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. Journal of Visualized Experiments. (51), e2720 (2011).
- Lee, W. G., Ortmann, D., Hancock, M. J., Bae, H., Khademhosseini, A. A hollow sphere soft lithography approach for long-term hanging drop methods. Tissue Engineering. Part C, Methods. 16 (2), 249-259 (2010).
- Timmins, N. E., Nielsen, L. K. Generation of multicellular tumor spheroids by the hanging-drop method. Methods in Molecular Medicine. 140, 141-151 (2007).
- de Souza, I. R., et al. Development of 3D cultures of zebrafish liver and embryo cell lines: a comparison of different spheroid formation methods. Ecotoxicology. 30 (9), 1893-1909 (2021).
- Ferreira, T., Rasband, W. ImageJ user guide. ImageJ/Fiji. 1, 151-161 (2012).
- Guidony, N. S., et al. ABC proteins activity and cytotoxicity in zebrafish hepatocytes exposed to triclosan. Environmental Pollution. 271, 116368 (2021).
- da Silva, N. D. G., et al. Interference of goethite in the effects of glyphosate and Roundup® on ZFL cell line. Toxicology In Vitro. 65, 104755 (2020).
- Yang, Y., et al. Temperature is a key factor influencing the invasion and proliferation of Toxoplasma gondii in fish cells. Experimental Parasitology. 217, 107966 (2020).
- Lopes, F. M., Sandrini, J. Z., Souza, M. M. Toxicity induced by glyphosate and glyphosate-based herbicides in the zebrafish hepatocyte cell line (ZF-L). Ecotoxicology and Environmental Safety. 162, 201-207 (2018).
- Lachner, D., Oliveira, L. F., Martinez, C. B. R. Effects of the water soluble fraction of gasoline on ZFL cell line: Cytotoxicity, genotoxicity and oxidative stress. Toxicology In Vitro. 30, 225-230 (2015).
- Morozesk, M., et al. Effects of multiwalled carbon nanotubes co-exposure with cadmium on zebrafish cell line: Metal uptake and accumulation, oxidative stress, genotoxicity and cell cycle. Ecotoxicology and Environmental Safety. 202, 110892 (2020).
- Dognani, G., et al. Nanofibrous membranes for low-concentration Cr VI adsorption: kinetic, thermodynamic and the influence on ZFL cells viability. Materials Research. , 24 (2021).
- ZEM2S (ATCC®CRL-2147™). American Type Culture Collection Available from: https://www.atcc.org/products/crl-2147 (2022)
- Bell, C. C., et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Scientific Reports. 6, 25187 (2016).
- Gajski, G., et al. Genotoxic potential of selected cytostatic drugs in human and zebrafish cells. Environmental Science and Pollution Research International. 23 (15), 14739-14750 (2016).
- Meng, Q., Yeung, K., Chan, K. M. Toxic effects of octocrylene on zebrafish larvae and liver cell line (ZFL). Aquatic Toxicology. 236, 105843 (2021).
- Mueller-Klieser, W. Method for the determination of oxygen consumption rates and diffusion coefficients in multicellular spheroids. Biophysical Journal. 46 (3), 343-348 (1984).
- Glicklis, R., Merchuk, J. C., Cohen, S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnology and Bioengineering. 86 (6), 672-680 (2004).
- Ho, R. K., Kimmel, C. B. Commitment of cell fate in the early zebrafish embryo. Science. 261 (5117), 109-111 (1993).
- Biswas, S., Emond, M. R., Jontes, J. D. Protocadherin-19 and N-cadherin interact to control cell movements during anterior neurulation. The Journal of Cell Biology. 191 (5), 1029-1041 (2010).
- Bradford, C. S., Sun, L., Collodi, P., Barnes, D. W. Cell cultures from zebrafish embryos and adult tissues. Journal of Tissue Culture Methods. 16 (2), 99-107 (1994).
- He, S., et al. Genetic and transcriptome characterization of model zebrafish cell lines. Zebrafish. 3 (4), 441-453 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved