Method Article
Open-Source Real-Time Closed-Loop Electrical Threshold Tracking for Translational Pain Research
In This Article
Summary
APTrack is a software plugin developed for the Open Ephys platform that enables real-time data visualization and the closed-loop electrical threshold tracking of neuronal action potentials. We have successfully used this in microneurography for human C-fiber nociceptors and mouse C-fiber and Aδ-fiber nociceptors.
Abstract
Nociceptors are a class of primary afferent neurons that signal potentially harmful noxious stimuli. An increase in nociceptor excitability occurs in acute and chronic pain conditions. This produces abnormal ongoing activity or reduced activation thresholds to noxious stimuli. Identifying the cause of this increased excitability is required for the development and validation of mechanism-based treatments. Single-neuron electrical threshold tracking can quantify nociceptor excitability. Therefore, we have developed an application to allow such measurements and demonstrate its use in humans and rodents. APTrack provides real-time data visualization and action potential identification using a temporal raster plot. Algorithms detect action potentials by threshold crossing and monitor their latency after electrical stimulation. The plugin then modulates the electrical stimulation amplitude using an up-down method to estimate the electrical threshold of the nociceptors. The software was built upon the Open Ephys system (V0.54) and coded in C++ using the JUCE framework. It runs on Windows, Linux, and Mac operating systems. The open-source code is available (https://github.com/
Introduction
Nociceptors are primary afferent neurons in the peripheral nervous system that are activated by overtly or potentially tissue-damaging events and play a critical protective role in acute pain1. Electrophysiological recordings from C-fiber and Aδ-fiber nociceptors in animal models, healthy human volunteers, and patients have revealed sensitization and abnormal spontaneous activity in a diverse range of pain conditions2,3,4,5,6,7. Understanding the mechanisms that underlie these changes in nociceptor excitability in patients could enable targeted therapeutic interventions8. However, there are few tools to assess nociceptor excitability directly, particularly in patients9, but the potential for the utility of such tools is well recognized10,11.
Whole-nerve electrical threshold tracking can be used to examine axonal excitability in humans12. However, as large, myelinated, peripheral neurons contribute disproportionally to the amplitude of the sensory compound action potential, whole-nerve electrical threshold tracking does not allow the assessment of C-fiber function11,13. Indeed, in a previous study, whole-nerve electrical threshold tracking in chronic neuropathic pain cohorts with diabetic neuropathy and chemotherapy-induced polyneuropathy showed no differences in axonal excitability11.
In a previous study, electrical threshold tracking at the single-neuron level was used to examine the excitability of C-fiber nociceptors during teased-fiber recordings in an ex vivo rat skin-nerve preparation14. The authors demonstrated that an increased potassium concentration, acidic conditions, and bradykinin all increased C-fiber nociceptor excitability, as reflected by a reduced electrical threshold for action potential generation. Furthermore, heating the receptive field of the heat-sensitive nociceptors reduced their electrical threshold, whereas heat-insensitive nociceptors exhibited an increase in their electrical threshold14. This provides important proof that single-neuron electrical threshold tracking is possible and can be of utility, but there are currently no software and/or hardware solutions available to enable such investigations, particularly for human studies.
In humans, microneurography is the only available method to directly assess the electrophysiological properties of C-fibers15. This approach has been used to demonstrate nociceptor dysfunction in patients with chronic pain2,3,4,5,6,7. Microneurography can detect single-neuron action potentials; however, due to the low signal-to-noise ratios, researchers use the marking technique to characterize C-fiber activity16. In the marking technique, suprathreshold electrical stimulation is applied to C-fiber receptive fields in the skin. This electrical stimulation generates an action potential that occurs at a constant latency, which is determined by the C-fiber's conduction velocity. C-fibers exhibit activity-dependent slowing, whereby their conduction velocity reduces and, therefore, their conduction latency increases during periods of action potential discharge17. Under basal conditions, C-fibers do not normally generate action potentials in the absence of noxious stimuli, and, therefore, their conduction latency in response to low-frequency electrical stimulation is constant. Mechanical, thermal, or pharmacological stimuli, which evoke firing, induce activity-dependent slowing, which increases the latency of the action potentials evoked by concomitant low-frequency electrical stimulation. This allows the objective identification of responses to the applied non-electrical stimuli in the context of a low signal-to-noise ratio. Therefore, activity-dependent slowing can be used to functionally characterize C-fibers16. Indeed, different functional classes of C-fibers exhibit distinctive patterns of activity-dependent slowing in electrical stimulation paradigms that involve varying the stimulation frequency18,19. This variability in the latency of C-fiber action potentials presents a challenge for algorithms designed to monitor them.
Ongoing activity in a nociceptor leads to increased variability in its latency during low-frequency electrical stimulation, and this is again due to activity-dependent slowing. This increased variability, or jitter, is a quantifiable proxy measure of excitability2. Further causes of variability in action potential latency include flip-flop, where alternate terminal branches of a single neuron are stimulated, which causes the evoked action potential to have two (or more) baseline latencies that are mutually exclusive20. Finally, changes in the temperature of a peripheral neuron's terminal branches also cause action potential latency changes in a thermodynamic manner, with warming increasing the conduction velocity and cooling slowing the conduction velocity19. Thus, any software seeking to perform closed-loop electrical threshold tracking of nociceptive C-fibers must allow for changes in latency in electrically evoked action potentials.
To achieve our goal of cross-species electrical threshold tracking of C-fiber nociceptors, we developed APTrack, an open-source software plugin for the Open Ephys platform21, to enable real-time, closed-loop, electrical threshold tracking, and latency tracking. We provide proof-of-concept data demonstrating that C-fiber nociceptor electrical threshold tracking during human microneurography is possible. Furthermore, we show that this tool can be used in rodent ex vivo teased-fiber electrophysiology, thus enabling translational studies between humans and rodents. Here, we will describe in detail how researchers can implement and use this tool to aid their study of nociceptor function and excitability.
Protocol
The human microneurography experiments were approved by the Faculty of Life Sciences Research Ethics Committee at the University of Bristol (reference number: 51882). All the study participants gave written informed consent. The animal experiments were performed at the University of Bristol in accordance with the UK Animals (Scientific Procedures) Act 1986 after approval by the University of Bristol Animal Welfare and ethical review board and were covered by a Project License.
1. Installing the Open Ephys GUI and APTrack
- Check the software documentation to find the most recent version of the Open Ephys graphical user interface (GUI) that is supported (https://github.com/Microneurography/APTrack#readme), and then download and install the GUI.
- Install a compatible version of the GUI from the following URL: https://github.com/open-ephys/plugin-GUI/releases.
- Download the latest version from GitHub: https://github.com/Microneurography/APTrack/releases. For a Windows computer, copy the .dll file into the plugins folder, which is usually found at C:\Program Files\Open Ephys\plugins. For a MacOS computer, copy the .bundle file into the Contents/PlugIns folder of the package.
2. Assembly of the recording and stimulating apparatus
- Connect the acquisition board to the computer using the manufacturer-supplied cable, and power it on.
NOTE: For human microneurography, a USB 3.0 isolator was used to electrically isolate the participant from the computer, and the acquisition board was powered by a portable battery as opposed to the mains voltage power supply used for rodent studies. All the USB connections, excluding the stepper motor control board, were passed through the USB isolator during the human studies. - Connect the I/O board to the analog in port on the acquisition board. Connect an Intan RHD recording headstage to the acquisition board using a serial-peripheral interface (SPI) cable.
NOTE: The Intan 16-channel bipolar headstage was used here, but other monopolar RHD2000 series headstages may be used. - Connect the PulsePal to the computer22. For assembly with an analog voltage-controlled stimulator (e.g., a DS4) using a PulsePal, as with the mouse teased-fiber recordings, follow steps 2.5.1-2.5.3; for assembly with a rotary encoder-based stimulator (e.g., a DS7) using a stepper motor, as with the human microneurography recordings, follow steps 2.6.1-2.6.8 (Figure 1).
- Build the signal chain in the GUI as described below.
- Insert the Rhythm FPGA plugin into the signal chain by left-clicking and dragging it into the signal chain; this connects the GUI to the acquisition board. Ensure the ADC button has been clicked on to initiate the recording of the ADC channels from the I/O board. The ADC button will light up orange when it is on.
NOTE: If you wish to play back previously recorded experimental data, the File Reader plugin can be used at the start instead of Rhythm FPGA. Using this in combination with APTrack will allow for the visualization and latency tracking of the action potentials in previous experiments. - Insert a bandpass filter into the signal chain; the default settings of 300-6,000 Hz are suitable for both human and mouse recordings. Additionally, insert a splitter after it.
- Insert the APTrack plugin into the signal chain on one side of the splitter and LFP Viewer on the other side. LFP Viewer provides a traditional oscilloscope-like voltage trace view, which is useful during experiments.
- Insert a record node after the plugin. In the drop-down menu, change the data save format from binary to Open Ephys. This completes a simple signal chain that works well (Figure 2); however, additional components can be added as determined by experimental requirements.
NOTE: If the record node is placed before the plugin in the signal chain, the action potential tracking information will not be saved. - At the top right of the GUI, click on the play button to begin transmitting data from the acquisition board and visualizing it. To begin recording, click on the circular record button next to the play button.
NOTE: It is easy to forget to click on record; we record data from the moment we begin acquiring to prevent this from happening.
- Insert the Rhythm FPGA plugin into the signal chain by left-clicking and dragging it into the signal chain; this connects the GUI to the acquisition board. Ensure the ADC button has been clicked on to initiate the recording of the ADC channels from the I/O board. The ADC button will light up orange when it is on.
- For assembly with an analog voltage-controlled stimulator, follow the steps as described below.
- Power on a constant current stimulator that has its stimulation amplitude controlled by an analog voltage input. A DS4 was used in this case (Figure 1).
- The PulsePal output channel 1 is for the analog voltage command. Split this signal using a BNC T-splitter, and then connect it to the constant current stimulator input and the I/O board so that the command voltage is recorded.
- The PulsePal output channel 2 is for the electrical stimulation TTL event marker. Connect this to the I/O board so that the stimulation TTL event markers are recorded for the plugin to use and for the post hoc analysis.
- For assembly with an analog voltage-controlled stimulator, follow the steps as described below.
- Power on a constant current stimulator that has its stimulation amplitude controlled by a rotary encoding dial. A DS7 was used in this case (Figure 1).
- Connect the stepper motor control board to the stepper motor using the manufacturer-supplied cable and magnetic mount.
- Connect the control board to the computer directly using any standard USB A to USB micro-B cable. Do not connect the control board on the participant side of the USB isolator as this is also connected to a 12 V mains power supply.
- If it is the first time using the control board, upload the stepper motor script from GitHub to the control board; this only needs to be done once, or if any software updates for the stepper motor script are released.
- Set the stimulation amplitude dial on the constant current stimulator to 0 mA. Use a custom mounting bracket to interface the stepper motor and the stimulation amplitude dial. These can be 3D-printed, which enables cheap, quick, and customizable mounting solutions. Consult GitHub to see if a mount has already been designed for the stimulator of choice.
- Use a custom barrel adapter to connect the stepper motor barrel to the stimulation amplitude control dial. These adapters should be constructed from metal for strength and durability reasons; however, 3D-printed parts would also be suitable, although they may need to be replaced regularly. Consult GitHub to see if a barrel adapter has already been designed for the stimulator of choice.
- Loosely attach the control board/stepper motor apparatus to the stimulator control dial using a custom mount and barrel adapter.
NOTE: The mount and barrel adapter will be tightened later once the software has been launched and the stepper motor, automatically, sets to position zero. - Connect the PulsePal as described in protocol steps 2.5.2-2.5.3 (minus connecting output channel 1 to a stimulator), as generating TTL event markers is still required for analysis and for the plugin to function. Additionally, connect output channel 2 to the DS7 stimulator to trigger it.
- Prepare the mouse skin-nerve preparation as described below.
- Provide C57BL/6J mice (Charles River Laboratories, UK, in this study) of 2-4 months of age and of both sexes with food and water ad libitum.
- After culling by anesthetic overdose through an intraperitoneal injection of sodium pentobarbital (≥200 mg/kg) and confirming the cessation of circulation, dissect the skin from the dorsal aspect of the mouse hind paw and the saphenous nerve, which innervates this area, using the methods described by Zimmermann et al.23.
- Maintain the skin-nerve preparation in carbogenated synthetic interstitial fluid (Table 1) at 30-32 °C in one-half of a custom-made dual-chamber acrylic bath (15 mL/min perfusion rate, 30 mL volume). Thread the nerve through a small hole into the mineral oil-filled chamber, and seal with petroleum jelly. The oil provides an insulated recording environment.
- Tease away two fine filaments from the trunk of the nerve using super fine forceps and hang one on each side of a bipolar silver/silver chloride recording electrode.
- Digitize and amplify the neural signal using an RHD2216 16-channel bipolar headstage, and process it using the acquisition board. Sample the signal at 30 kHz, with a bandpass filter of 300-6,000 Hz, and visualize it using the GUI.
- Using a blunt glass rod, stroke the skin of the preparation. Use the low-amplitude mass activity to confirm that the preparation is alive.
- Perform human C-fiber microneurography as described below.
- Conduct microneurography with participants who have provided written informed consent, as previously described24.
- With the participant seated reclined comfortably on a bed and supported with pillows, identify the superficial peroneal nerve using an ultrasound scanner, and mark a target area approximately 5-10 cm proximal to the lateral malleolus, around the mid-shin level.
- Sterilize the skin around the target area using a 2% chlorhexidine in 70% alcohol wipe and insert a sterile reference electrode subcutaneously near the intended recording site at the mid-shin level.
- Insert a sterile recording electrode into the superficial peroneal nerve under ultrasound guidance within the target area.
- Digitize and amplify the neural signal using an RHD2216 16-channel bipolar headstage, and process it using the acquisition board. Sample the signal at 30 kHz, with a bandpass filter of 300-6,000 Hz, and visualize it using the GUI.
NOTE: The acquisition equipment was electrically isolated from the laptop by a USB 3.0 isolator with 5 kV RMS isolation and powered via a custom-made 12 V battery power supply. - Confirm successful intraneural positioning by gently stroking the skin to reveal mechanically evoked mass activity. Additionally, participants usually report paresthesia in the dorsolateral aspect of the foot upon successful intraneural positioning.
3. Software setup and identification and phenotyping of peripheral neurons
- Set up the software as described below.
- Open the GUI (Figure 3). If the stepper motor control board is connected to your PC, it will be detected and set itself to position zero. Tighten the custom mount and barrel adapter described in steps 2.6.5-2.6.7, as the stimulator's stimulation amplitude dial and stepper motor are both set to zero.
NOTE: If the stepper motor and stimulation amplitude dial are not both "zeroed", this can result in the stepper motor trying to turn the control dial out of its range, which may cause damage. - In the options menu, select the Trigger Channel. Choose the ADC channel containing the electrical stimulation TTL marker from the PulsePal output channel 2.
- In the options menu, select the Data Channel, and choose the channel containing the electrophysiological data.
- In the stimulation control panel, define the initial, minimum, and maximum stimulation amplitudes using the slider. Ensure the current stimulation is set above 0 so that TTL markers are generated.
NOTE: Some stimulators have an input-to-output scaling ratio that is not 1:1; consider this when selecting an appropriate stimulation amplitude. For example, a 1:10 output ratio can be selected on some stimulation systems to achieve a higher output from the constant current stimulator. - In the stimulation control panel, click on F to load a file containing the stimulation instructions. Electrical stimulation protocols are stored as comma-separated value (CSV) files composed of the desired stimulation frequencies and duration, which allows users to create complex stimulation paradigms for their experiments. An example template is available here: https://github.com/Microneurography/APTrack/blob/main/example_playlist.csv
- In the stimulation control panel, click on > to begin the loaded stimulation paradigm. By default, APTrack requests the PulsePal to generate 0.5 ms duration positive square wave pulses of varying amplitudes to control the constant current stimulator's stimulation amplitude.
- The temporal raster plot will begin updating with the response to electrical stimulation, with each new stimulation response being displayed as a new column on the right.
- Open the GUI (Figure 3). If the stepper motor control board is connected to your PC, it will be detected and set itself to position zero. Tighten the custom mount and barrel adapter described in steps 2.6.5-2.6.7, as the stimulator's stimulation amplitude dial and stepper motor are both set to zero.
- Visualize and identify single-neuron action potentials.
- For the successful detection of single-neuron action potentials, it is important to have suitable image thresholds set. In the temporal raster plot panel, adjust the low, detection, and high image threshold values.
- Select a color scheme in the options menu. In WHOT (White Hot) mode (default), voltages below the low image threshold are encoded in black. Voltages between the low image and detection thresholds are encoded in greyscale. Voltages above the detection threshold are encoded in green, and voltages above the high image threshold are encoded in red.
- Peripheral neurons exhibit constant latency responses at low stimulation frequencies (<0.25 Hz), and these responses are determined by their conduction velocity and the distance between the stimulation and recording sites. With suitable image thresholds set, the threshold crossing events detected by the algorithms will be encoded in green (Figure 4).
- Systematically move the stimulating electrode around the skin area innervated by the nerve that is being recorded, allowing for a minimum of three stimulation events at each site. Monitor the temporal raster plot for threshold crossing events (marked in green) occurring at the same time point after each electrical stimulation event.
NOTE: In mice, a search stimulus of 5 mA was used. In humans, the amplitude of the transcutaneous electrical search stimulus was titrated to a verbal pain rating such that it never exceeded 7/10. - Check for three threshold crossing events (green bars) that appear in a row at the same latency and in the same stimulation position; this indicates the identification of a peripheral neuron action potential.
- Optimize the stimulating electrode position by identifying the most electrically sensitive point of the target neuron's receptive field, and then fix the electrode in position. At this point in human microneurography, switch to using intradermal electroacupuncture needles (0.2 mm diameter) for bipolar electrical stimulation, in mice, a custom transcutaneous stimulating probe is used so that the stimulation position is constant.
- For the successful detection of single-neuron action potentials, it is important to have suitable image thresholds set. In the temporal raster plot panel, adjust the low, detection, and high image threshold values.
- Perform classification and sensory phenotyping of the peripheral neurons.
- Estimate the electrical threshold of the target action potential by adjusting the simulation amplitude manually or by using APTrack if desired (described in steps 4.1-4.2).
- Stimulate the receptive field at 2x the estimated electrical threshold at a frequency of 0.25 Hz throughout the sensory phenotyping protocol.
- Calculate the conduction velocity of the neuron by dividing the conduction distance by the conduction latency. C-fibers can be identified by a conduction velocity of ≤2 m/s.
- Mechanically stimulate the receptive field using von Frey filaments to determine the mechanical threshold for activation. Mechanosensation can be identified by evoked action potentials visible on the voltage trace and an increase in the latency of the neuron, if it is a C-fiber, upon sufficient force.
- Heat the receptive field of the neuron, again watching for action potentials visible on the voltage trace and an increase in the latency of the neuron, if it is a C-fiber, upon sufficient heat application. Heat-insensitive neurons will exhibit a decrease in latency due to the thermodynamic effect on axonal propagation.
NOTE: In human microneurography, use a TSC-II for rapid and accurate thermal control. In the mouse preparation, add warmed or cooled synthetic interstitial fluid to an aluminum isolation chamber placed over the receptive field to allow access to the neuron terminals while restricting rapid heat dissipation into the surrounding fluid. Record the temperature using a thermocouple. - Cool the receptive field, again watching for action potentials visible on the voltage trace and a marked increase in the latency of the neuron, if it is a C-fiber, upon sufficient cold application. All neurons will exhibit an increase in latency due to the thermodynamic effect on axonal propagation, so exercise caution in labeling neurons as cold-sensitive based on a latency increase alone.
4. Latency and electrical threshold tracking
- Perform latency tracking as described below.
- Following the identification of single-neuron action potential(s) on the temporal raster plot, move the gray linear slider on the right side of the temporal raster plot to adjust the position of the search box.
- Below the temporal raster plot, adjust the search box width rotary slider to an appropriate width. Make the search box width narrow to reduce the chance of transient noise spikes, spontaneously firing action potentials, or other nearby constant latency action potentials being misidentified as the action potential of interest.
- To begin tracking the targeted action potential, click on the + below the multi-unit tracking table. A new row will be added to the table containing details of the target action potential, including the latency location, the percentage firing over 2-10 stimuli (adjusted in the options menu), and the peak amplitude detected.
- Once an action potential is added to the multi-unit tracking table, the latency tracking algorithm (Figure 5) will automatically be executed on it upon every subsequent electrical stimulation.
- If there are multiple discrete action potentials visible on the temporal raster plot, add them to the multi-unit tracking table as described above. The theoretical maximum number of action potentials that can be added to the table for simultaneous latency tracking is the maximum 32 bit integer value.
- Check the Track Spike box in the multi-unit tracking table to move the search box to the appropriate position for that particular action potential, as determined by the latency tracking algorithm. This will allow for monitoring the latency tracking in real time and ensure that the tracking is following the action potential as expected. The latency tracking of other spikes will continue as normal in the background.
- Remove tracked action potentials from the multi-unit tracking table using the delete button at the end of each row.
- Perform electrical threshold tracking as described below.
- Adjust the increment and decrement rates in the stimulation control panel between 0.1 V and 0.5 V. Keep these values equal, and do not adjust them during the experiment unless this is part of the experimental paradigm.
- Ensure the stimulation frequency is set to an appropriate rate, typically 0.25-0.5 Hz, unless modulation of stimulation frequency is part of the experimental paradigm. Increasing nociceptor firing rates may alter the electrical threshold of nociceptor.
- Once an action potential is being successfully tracked, check the Track Threshold box in the multi-unit tracking table, which will initiate the electrical threshold tracking algorithm (Figure 6).
NOTE: Electrical threshold tracking is only run on the targeted action potential; indeed, the firing rates of other action potentials in the multi-unit tracking table will be updated accordingly as the stimulation amplitude changes. - Adjust the stimulation amplitude manually to the estimate of the electrical threshold; this will reduce the waiting time to determine the electrical threshold. The time taken to establish a reliable electrical threshold is dependent on the stimulation frequency, the increment and decrement rates, and the difference in stimulation amplitude from the initial stimulation to the electrical threshold of the neuron.
- The software uses an up-down method for the estimation of the electrical threshold of the neurons. In the multi-unit tracking table, the firing rate is determined over 2-10 previous stimulations (selected in the options menu). Select the number of stimulation events to be considered; a higher number will increase the reliability of the threshold estimate but will take longer to achieve.
- During human microneurography, it is important to monitor the painfulness of electrical stimuli to prevent excessive participant discomfort; some discomfort is unavoidable during the study of nociceptors, particularly of silent/sleeping C-fibers. Regularly ask for pain ratings while the stimulation amplitude increases during the electrical threshold tracking and remain nearby the constant current stimulator to disengage it upon the participant's request.
NOTE: Alternatively, the electrical stimulation can be disengaged through the user interface by clicking on the [ ] button in the stimulation control panel. - A firing rate of 50% indicates the approximate electrical threshold has been determined.
- While electrical threshold tracking, apply an experimental manipulation to the receptive field, such as temperature or drug manipulations. The effects of these manipulations on the electrical threshold of the nociceptor will be tracked.
NOTE: Allow sufficient time to identify a new nociceptor threshold following the experimental manipulation.
Results
A representative example of the software working to control an experiment is shown in Figure 7. It iteratively adjusts the stimulation amplitude using an up-down method to effectively find the electrical threshold of single nociceptors. For the first time, we demonstrate the feasibility of real-time single-neuron electrical threshold tracking in humans during microneurography (Figure 7A). Additionally, we show electrical threshold tracking in a mouse Aδ-fiber (Figure 7B). The identification of action potentials by threshold crossing, as used here, is sufficient for tracking electrical thresholds over time. We recommend users take steps to minimize electrical noise during their recordings, such as by using a Faraday cage and bandpass filters to improve the signal-to-noise ratio.
To demonstrate that electrical threshold tracking can be used as a measure of changes in nociceptor excitability in humans, tracking of the electrical threshold during a stepped heating paradigm was performed (Figure 8). Increasing the temperature of the nociceptor terminals decreased the electrical stimulation current required to elicit an action potential, reflecting an increase in nociceptor excitability (Figure 8C). This was likely caused by the generation of receptor potentials by the heat-sensitive ion channels expressed in the C-fiber nociceptor14. At the highest temperature step, 44 °C, thermally evoked action potentials were elicited (Figure 8A, stimulus number 86-96). This causes an increase in the electrical threshold as the nociceptor may be in a refractory state following high-frequency discharge. As expected, the latency of the tracked action potential decreased as the temperature increased. This is thought to occur due to a thermodynamic effect on the conduction machinery, which increases the conduction velocity of the C-fiber. This C-fiber may also be exhibiting flip-flop (Figure 8B, stimulus number 47-54), which can result in the following electrical stimulation being erroneously increased in amplitude if the action potential falls outside of the algorithm search window.
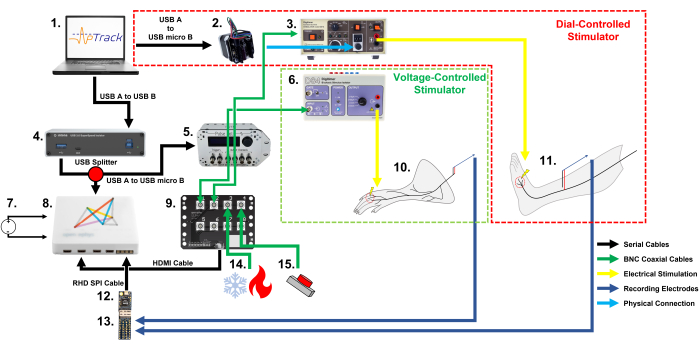
Figure 1: A schematic of the equipment setup and cable connections required for nociceptor electrical threshold tracking with APTrack in rodents and humans. Note the two different methods of stimulation amplitude controls: a stepper motor for manually adjusted stimulators in our human setup, and a PulsePal for input voltage-controlled stimulators in our rodent setup. (1) A PC (Windows, Mac, or Linux) running the plugin for the Open Ephys platform. (2) A stepper motor that operates the stimulation amplitude dial on the DS7. (3) A constant current stimulator approved for use in humans; here we used a DS7. (4) A USB 3.0 optoisolator, which isolates the human participant from the PC (optional, only required for human research). (5) A PulsePal V2 Pulse Generator, which generates TTL time stamps (output channel 2) and voltage steps corresponding to the requested stimulation amplitude (output channel 1). (6) A constant current stimulator for use in animals; here, we used a DS4. (7) A DC power supply for the system (mains DC power supply used for the rodent setup and battery DC power supply used for the human setup). (8) An acquisition board. (9) An I/O board to connect the BNC coaxial cables carrying the signals to be recorded, such as the thermocouple outputs and TTL markers. (10) The mouse skin-nerve preparation undergoing nociceptor electrophysiological recordings. (11) A human participant undergoing microneurography recording from C-fibers in the superficial peroneal nerve. (12) An Intan RHD2216 headstage for the acquisition and digitization of the recordings. (13) An Intan Electrode Adapter Board, which the recording electrodes are connected to and which allows the signal to be passed to the RHD2216 headstage. (14) A thermal stimulation system that can output the temperature via a BNC coaxial connection. (15) A 3.3 V battery-powered button/foot pedal that is used for marking the mechanical stimulation events and drug applications. Please click here to view a larger version of this figure.
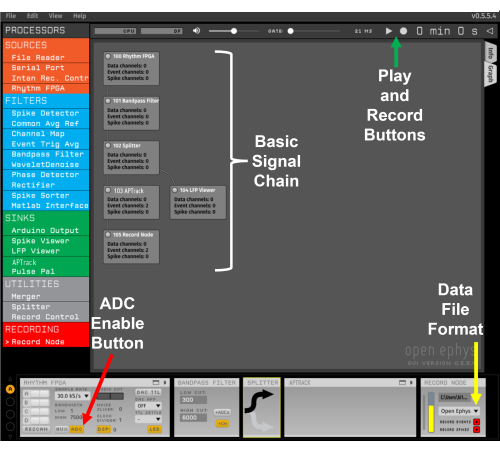
Figure 2: Template signal chain. The red arrow points to the button for enabling the ADC input from the I/O board. The yellow arrow indicates the drop-down menu for selecting the Open Ephys file format. The green arrow indicates the Play and Record buttons. Please click here to view a larger version of this figure.
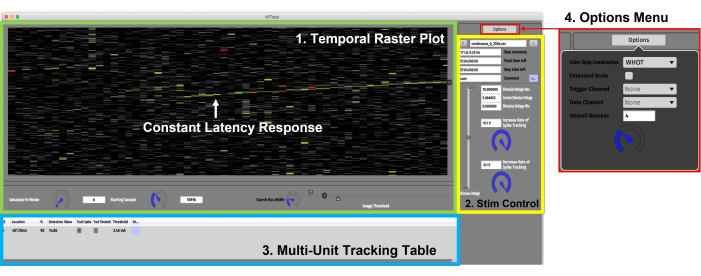
Figure 3: Graphical user interface. The GUI consists of four main components. (1) Temporal Raster Plot panel (green) for data visualization and the settings associated with controlling the plot. A constant latency response showing gradual activity-dependent slowing is indicated by the green arrow. (2) Stimulation Control panel (yellow) for setting the stimulation amplitude parameters and loading the stimulation paradigm scripts. (3) Multi-Unit Tracking Table (blue) for adding the action potentials for tracking and activating the latency and electrical threshold tracking. (4) Options Menu for selecting the color styles and the input channel for the data and TTL triggers. Please click here to view a larger version of this figure.
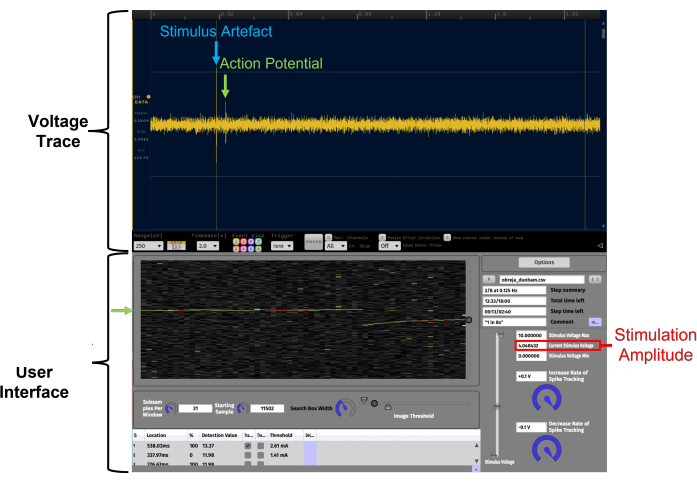
Figure 4: Facilitation of the identification of constant latency action potentials through real-time data visualization on a temporal raster plot using APTrack. This is a high signal-to-noise ratio example. The data presented in the temporal raster plot are from a human C-fiber recording from the superficial peroneal nerve during microneurography. Voltage Trace is the oscilloscope-like LFP Viewer plugin within Open Ephys. The APTrack User Interface is the graphical user interface of the plugin. The tracked action potential is indicated by green arrows, and the circular slider on the border of the temporal raster plot is for controlling the search box position where the algorithms will search for threshold crossing events. The electrical stimulation artifact is marked in blue on the voltage trace. The stimulation amplitude of the analog voltage command is indicated in red; note that this may not be the same as the stimulation current amplitude depending on the scaling factor set on the stimulator. Please click here to view a larger version of this figure.
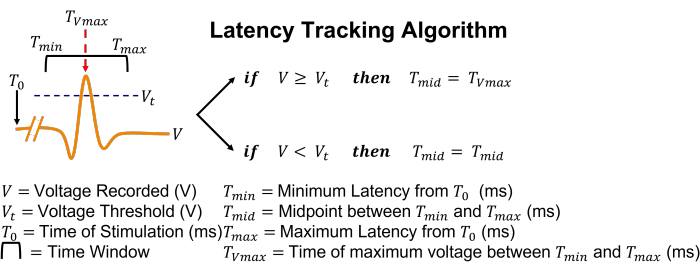
Figure 5: Graphical representation of the latency tracking algorithm. In simple terms, if an action potential is detected by threshold crossing, the search box will adjust its position to center itself at the time of the peak voltage. Please click here to view a larger version of this figure.

Figure 6: Graphical representation of the electrical threshold tracking algorithm. In simple terms, if an action potential is detected by threshold crossing, the stimulation amplitude will be decreased by the decrement rate. If no action potential is detected, the stimulation amplitude will be increased by the increment rate. Please click here to view a larger version of this figure.
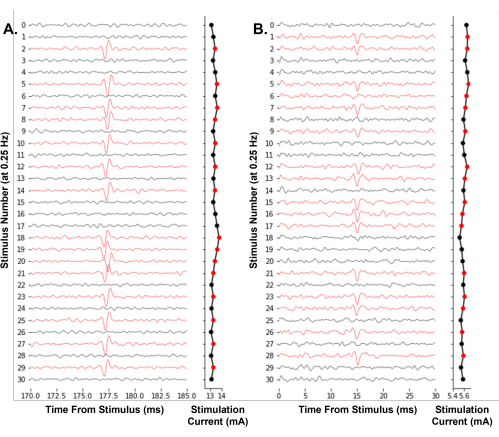
Figure 7: Automated electrical threshold tracking of single-neuron action potentials at a 0.25 Hz stimulation frequency. (A) Sequential traces of a human C-fiber of the superficial peroneal nerve during a microneurography experiment. (B) Sequential traces of a mouse Aδ-fiber of the saphenous nerve during skin-nerve preparation teased fiber electrophysiology. The traces were colored red when an action potential was identified, resulting in a decrease in the stimulus amplitude. The software algorithm effectively finds the stimulus amplitude required for a 50% likelihood of firing. Please click here to view a larger version of this figure.
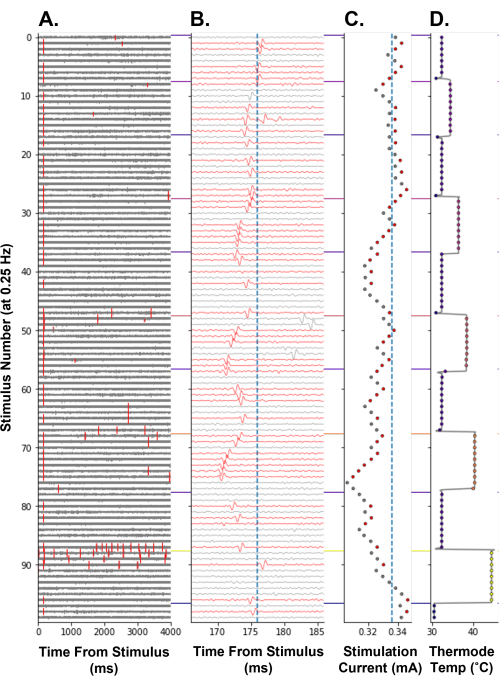
Figure 8: Electrical threshold tracking at a 0.25 Hz stimulation frequency during the thermal stimulation of a human C-fiber nociceptor. The y-axis encodes the stimulation number from the start of the paradigm. (A) Voltage trace for 4,000 ms following electrical stimulation, with threshold crossing events marked in red. (B) Voltage trace from A zoomed in around the tracked action potential. The traces were colored red when the tracked action potential was detected. The vertical blue line is the baseline latency of the tracked unit. (C) Stimulation current commanded by APTrack. The vertical blue line is the baseline electrical threshold. (D) Receptive field TCS-II thermal stimulating probe temperature. Please click here to view a larger version of this figure.
| Compound | Concentration |
| NaCl | 107.8 mM |
| NaHCO3 | 26.2 mM |
| KCl | 3.5 mM |
| NaH2PO4 | 1.67 mM |
| CaCl2 | 1.53 mM |
| MgSO4 | 0.69 mM |
| Sodium Gluconate | 9.64 mM |
| Sucrose | 7.6 mM |
| Glucose | 5.55 mM |
Table 1: Contents of the synthetic interstitial fluid for the mouse skin-nerve preparation23.
Discussion
APTrack is a software plugin for use with the Open Ephys platform. We have chosen this platform as it is open-source, flexible, and cheap to implement. Not including the cost of the constant current stimulator, all the equipment required to start using the plugin could be purchased for around $5,000 USD at the time of writing. We hope this will enable researchers to implement APTrack in their peripheral nerve electrophysiology studies more easily. Furthermore, researchers can freely modify the software to fit their experimental needs. Importantly, this tool has allowed the electrical threshold tracking of single C-fiber nociceptors, for the first time, in humans.
The higher the signal-to-noise ratio, the better the algorithms can identify action potentials. The signal-to-noise ratio during microneurography was sufficient in the majority of our recordings, but users must be alert to the risk of signal degradation over time. This is particularly important for longer experimental protocols, because if the tracked action potential's amplitude drops below the detection threshold, the stimulation amplitude will be increased mistakenly; this can be mitigated by experimenters monitoring the plugin and then adjusting the settings if required. The signal-to-noise ratio is improved with bandpass filtering, but larger transients may still be misidentified as action potentials should they arrive during the search box time window. The risk of misidentifying transient noise as an action potential can be reduced by narrowing the time window during which the plugin searches for action potentials and by optimizing the threshold settings. However, there are still situations one may encounter that impede the plugin's performance. Spontaneous activity may cause difficulties if larger-amplitude action potentials fall within the algorithm's search box window, as they will be misidentified as the target action potential. Additionally, spontaneous activity in the neuron of interest may mean that the electrical stimulation falls during its refractory period, causing failure to generate an action potential. Difficulties using the software can also arise when primary afferent neurons exhibit flip-flop, whereby alternate terminal branches of a single neuron are stimulated, thus causing the evoked action potential to have two (or more) baseline latencies that are mutually exclusive20. During recordings from neurons exhibiting flip-flop with high signal-to-noise ratios, we successfully performed latency and electrical threshold tracking by increasing the search box width to encapsulate all the potential conduction velocities that the neuron exhibited. However, the electrical threshold may vary depending on the terminal branch of the neuron that is being excited, which is likely in part due to differences in the distance from the site of the electrical stimulation to the alternate nociceptor terminals. Additional work on the action potential identification process to include, for example, template matching is feasible and could be integrated into this software. The GUI plugins for band-stop or adaptive noise filtration could also be used upstream of APTrack in the signal chain should they be developed.
We consider the electrical threshold determined to be the current required to elicit an action potential 50% of the time, over a user-defined number of electrical stimuli, typically 2-10. The morphology of electrical stimulation is 0.5 ms and positive, square wave pulses. This is not the same as determining the rheobase, a commonly used measure of neuronal excitability. The plugin could be adapted to determine the rheobase. However, we pursued a simpler measure, as dynamic changes in excitability, such as those hypothesized to occur during heating, would have been more difficult to quantify with rheobase changes than our electrical threshold estimate.
This software can be used in both human and rodent experiments. This is made possible by flexible support for the electrical stimulation systems. The software will work with any stimulator that accepts an analog command voltage or can be manually interfaced with a stepper motor. For microneurography, we used it with a CE-marked constant current stimulator that was designed for use in human research and had its stimulation controlled by a dial. Stimulators that accept analog voltage commands can be noisy as they do not disconnect the circuit between stimuli, meaning any 50/60 Hz hum or noise on the analog input will be transmitted to the recording. A stimulator that requires an additional TLL trigger signal to connect the circuit, permitting a stimulus at a current analogous to the analog voltage input to be generated, is ideal for use with the plugin. This prevents the noise from being transmitted to the recording between stimuli.
The software uses a simple up-down method to estimate the electrical threshold. This has been used in psychophysics tests for many decades25. In line with the up-down method, the electrical threshold tracking algorithm for modulating the stimulation amplitude only considers the previous stimulation's amplitude and response when calculating the next stimulation's amplitude. This means that the stimulation amplitude will oscillate around the true electrical threshold, thus producing a 50% firing rate, assuming the threshold is stable. The minimum size of an increment or decrement is 0.01 V; this is equivalent to 0.01 mA assuming the stimulator has a 1 V:1 mA input-to-output ratio and sufficient resolution to achieve step changes this small. The plugin will update the live estimate of the target action potential's electrical threshold every time it reaches a 50% firing rate over a user-defined number of previous stimuli (2-10). Post hoc, we recommend using a rolling average of the stimulation amplitude over the last 2-10 stimuli to estimate the electrical threshold, and it should be noted that this estimate will only be accurate when the firing rate is relatively stable at 50%. In both the live and post hoc estimates of the electrical threshold, there is a balance of resolution, reliability, and time to consider. Using smaller increment and decrement steps will increase the accuracy of the electrical threshold estimate but will increase the time taken to find the new electrical threshold initially and following perturbation. Calculating the electrical threshold over a greater number of previous stimuli will provide better reliability but will increase the time required to reach an accurate estimate.
APTrack was designed for use in peripheral nerve recordings, specifically to track the electrical thresholds of C-fibers during experimental and pathological perturbations over periods where the action potential latency may vary depending on the underlying neuronal activity. This method will enable the examination of not only axonal excitability but also of nociceptor generator potentials in healthy volunteers and patients. We anticipate that other fields of electrophysiology may adopt and adapt this tool for use in any experiment that requires the electrical threshold tracking of a stimulus-locked activity. For example, this could as easily be adapted for optogenetic stimulation with light pulses driven from APTrack. The plugin is open-source and available to researchers under a GPLv3 license. It is built on the Open Ephys platform, which is an adaptable, low-cost, open-source data acquisition system. The plugin provides additional hooks for downstream plugins to extract the action potential information and provide additional user interfaces or adaptative paradigms. The plugin provides a simple user interface for the visualization and latency tracking of action potentials in real time. It can also play back previous data and visualize them using the temporal raster plot. Furthermore, it can also perform latency tracking during the playback of previous data. While there are other software packages available for real-time latency tracking, they are not open-source and cannot perform electrical threshold tracking26,27. APTrack has an advantage over traditional methods of identifying constant latency action potentials from voltage traces as it uses a temporal raster plot for the data visualization. Furthermore, our experiences of using it in experiments with low signal-to-noise ratios have indicated that the temporal raster plot visualization method allows the identification of constant latency action potentials that may have been otherwise missed.
Whole-nerve threshold tracking is a widely used method for assessing axonal excitability13. Single-neuron electrical threshold tracking in rodent C-fibers has been used previously to quantify nociceptor excitability14, and its utility in humans is recognized10,11; however, until now, this was not possible. We provide a novel, open-source tool to directly measure single nociceptor excitability in both rodent and human peripheral nerve electrophysiological studies. APTrack enables the real-time, open-source, electrical threshold tracking of single-neuron action potentials in humans, for the first time. We anticipate that it will facilitate translational studies of nociceptors between rodents and humans.
Disclosures
G.W.T.N. is a BBSRC Collaborative Training Partnership Doctoral Studentship with the University of Bristol and Eli Lilly and Company (BB/T508342/1). A.P.N. is a current employee of Eli Lilly and Company and may own stock in this company.
Acknowledgements
We would like to thank our funders for their support: Academy of Medical Sciences (J.P.D., A.E.P.), Versus Arthritis (J.P.D., A.E.P.), Jean Golding Institute Seedcorn Grant (J.P.D., A.E.P., G.W., A.C.S., M.M.P.), and Biotechnology and Biological Sciences Research Council collaborative training partnership doctoral studentship with Eli Lilly (G.W.T.N.). We would like to extend our thanks to all the contributors to the development of APTrack. We would also like to thank our volunteers who participated in the microneurography experiments and our Patient and Public Involvement and Engagement collaborators for their invaluable contributions.
Materials
| Name | Company | Catalog Number | Comments |
| 12V DC Power Supply | NA | NA | To power uStepper S-lite. Required for dial-controlled stimulators. |
| 36 Pin Electrode Adapter Board | Intan Technology | C3410 | APTrack Dependency. For connecting electrode input to headstage. $255 USD as of March 2021. |
| APTrack Plugin | NA | NA | https://github.com/Microneurography/APTrack |
| Bipolar Ag/AgCl Recording Electrode | Custom | NA | Recording electrode for the skin-nerve preparation. Or equivalent. |
| Bipolar Concentric Stimulating Electrode | World Precision Instruments | SNE-100 | For electrical stimulation in the mouse skin-nerve preparation. Or equivalent. |
| Bipolar Transcutaneous Stimulating Electrode | Custom | NA | For transcutaneous electrical stimulation while searching for single-neuron action potentials during microneurography. |
| BNC T Splitter (1+) | NA | NA | APTrack Dependency. Any standard BNC T splitter. |
| BNC to BNC cables (3+) | NA | NA | APTrack Dependency. Any standard BNC cables. |
| C6H11NaO7 | Merck | S2054 | Skin-nerve preparation synthetic interstitial fluid constituent. Or equivalent. |
| CaCl2 | Merck | C5670 | Skin-nerve preparation synthetic interstitial fluid constituent. Or equivalent. |
| Digitimer DS4 Constant Current Stimulator | Digitimer | DS4 | Constant current stiulator for animal research. £1,695 GBP as of September 2022. |
| Digitimer DS7 Constant Current Stimulator | Digitimer | DS7A | Constant current stiulator for human research. £3,400 GBP as of September 2022. |
| Electroaccupuncture Classic Plus Stimulating Electrodes | Harmony Medical | NA | For fixed position intradermal electrical stimulation of the dorsal aspect of the foot during human microneurography. |
| Glucose | Fisher Scientific | G/0450/60 | Skin-nerve preparation synthetic interstitial fluid constituent. Or equivalent. |
| HDMI Cable | NA | NA | APTrack Dependency. Any standard passive HMDI cable. To connect OE I/O Board to OE Acquisition Board. |
| KCl | Merck | P9541 | Skin-nerve preparation synthetic interstitial fluid constituent. Or equivalent. |
| MgSO4 | Acros Organics | 213115000 | Skin-nerve preparation synthetic interstitial fluid constituent. Or equivalent. |
| Mineral Oil | Merck | 330779 | Electrical insulation for nerve recordings in th skin-nerve preparation. Or equivalent. |
| NaCl | Merck | S9888 | Skin-nerve preparation synthetic interstitial fluid constituent. Or equivalent. |
| NaHCO3 | Merck | S6014 | Skin-nerve preparation synthetic interstitial fluid constituent. Or equivalent. |
| NaHCO3 | Merck | S0751 | Skin-nerve preparation synthetic interstitial fluid constituent. Or equivalent. |
| Open Ephys Acquisition Board | Open Ephys | NA | APTrack Dependency. Includes USB cable to connect to computer and mains socket power supply. €2,955 EUR as of September 2022. |
| Open Ephys Graphical User Interface | Open Ephys | NA | https://github.com/open-ephys/plugin-GUI |
| Open Ephys I/O Board | Open Ephys | NA | APTrack Dependency. For ADC voltage inputs via BNC cables. €12.5 EUR without connectors, €85 EUR with connectors as of September 2022. |
| PulsePal V2 | Sanworks | 1102 | APTrack Dependency. Open-source DAC and train generator. $725 USD pre-assembled as of September 2022. Approx. $275 USD for self-assembly. |
| RHD 6ft SPI Cable | Intan Technology | C3206 | APTrack Dependency. For connecting headstage to OE Acquisition Board. $295 USD as of March 2021 |
| RHD2216 16ch Bipolar Headstage | Intan Technology | C3313 | APTrack Dependency. For data acquisition and digitization. $725 USD as of March 2021. Or equivalent RHD2000 series headstage. |
| Sucrose | Fisher Scientific | S/8560/60 | Skin-nerve preparation synthetic interstitial fluid constituent. Or equivalent. |
| TCS-II Thermal Stimulator | QST.Lab | NA | For thermal stimualtion of nociceptor receptive fields during human microneurography. |
| Tungsten Microelectrode Pair (Active + Reference) | FHC | 30085 | For microneurography recordings. 35mm. |
| Ultrasound Scanner iQ+ | Butterfly Network | NA | For ultrasound-guided electrode insertion during microneurography. |
| USB 3.0 5kV RMS Isolation | Inota Technology | 7055-D | For isolating human microneuroography participant from computer. €459 EUR as of September 2022. |
| USB-A to micro USB-B cable (2) | NA | NA | APTrack Dependency. To connect computer to PulsePal and to uStepper S-lite if using stepper-stimulator interfacing. |
| uStepper S-lite + NEMA17 motor | uStepper | NA | To interface with stimulators via a control dial. €50 EUR as of September 2022. |
| Von Frey Filaments | Ugo Basile | 37450-275 | For mechanical stimulation of receptive fields during sensory phenotyping of nociceptors. |
References
- Dubin, A. E., Patapoutian, A. Nociceptors: The sensors of the pain pathway. Journal of Clinical Investigation. 120 (11), 3760-3772 (2010).
- Serra, J., et al. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 153 (1), 42-55 (2012).
- Serra, J., et al. Hyperexcitable C nociceptors in fibromyalgia. Annals of Neurology. 75 (2), 196-208 (2014).
- Namer, B., et al. Specific changes in conduction velocity recovery cycles of single nociceptors in a patient with erythromelalgia with the I848T gain-of-function mutation of Nav1.7. Pain. 156 (9), 1637-1646 (2015).
- Kleggetveit, I. P., et al. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain. 153 (10), 2040-2047 (2012).
- Orstavik, K., et al. Abnormal function of C-fibers in patients with diabetic neuropathy. Journal of Neuroscience. 26 (44), 11287-11294 (2006).
- Orstavik, K., et al. Pathological C-fibres in patients with a chronic painful condition. Brain. 126, 567-578 (2003).
- Raja, S. N., Ringkamp, M., Guan, Y., Campbell, J. N., John, J. Bonica Award Lecture: Peripheral neuronal hyperexcitability: The "low-hanging" target for safe therapeutic strategies in neuropathic pain. Pain. 161, S14-S26 (2020).
- Middleton, S. J., et al. Studying human nociceptors: From fundamentals to clinic. Brain. 144 (5), 1312-1335 (2021).
- Marshall, A., Alam, U., Themistocleous, A., Calcutt, N., Marshall, A. Novel and emerging electrophysiological biomarkers of diabetic neuropathy and painful diabetic neuropathy. Clinical Therapeutics. 43 (9), 1441-1456 (2021).
- Themistocleous, A. C., et al. Axonal excitability does not differ between painful and painless diabetic or chemotherapy-induced distal symmetrical polyneuropathy in a multicenter observational study. Annals of Neurology. 91 (4), 506-520 (2022).
- Bostock, H., Cikurel, K., Burke, D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 21 (2), 137-158 (1998).
- Kiernan, M. C., et al. Measurement of axonal excitability: Consensus guidelines. Clinical Neurophysiology. 131 (1), 308-323 (2020).
- Sauer, S. K., et al. Can receptor potentials be detected with threshold tracking in rat cutaneous nociceptive terminals. Journal of Neurophysiology. 94 (1), 219-225 (2005).
- Vallbo, A. B. Microneurography: How it started and how it works. Journal of Neurophysiology. 120 (3), 1415-1427 (2018).
- Torebjork, H., Hallin, R. A new method for classification of C-unit activity in intact human skin nerves. Advances in Pain Research and Therapy. 1, 29-34 (1976).
- Brown, G. L., Holmes, O. The effects of activity on mammalian nerve fibres of low conduction velocity. Proceedings of the Royal Society of London. Series B: Biological Sciences. 144 (918), 1-14 (1956).
- Obreja, O., et al. Patterns of activity-dependent conduction velocity changes differentiate classes of unmyelinated mechano-insensitive afferents including cold nociceptors, in pig and in human. Pain. 148 (1), 59-69 (2010).
- Serra, J., Campero, M., Ochoa, J., Bostock, H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. Journal of Physiology. 515, 799-811 (1999).
- Weidner, C., Schmidt, R., Schmelz, M., Torebjork, H. E., Handwerker, H. O. Action potential conduction in the terminal arborisation of nociceptive C-fibre afferents. Journal of Physiology. 547, 931-940 (2003).
- Siegle, J. H., et al. Open Ephys: An open-source, plugin-based platform for multichannel electrophysiology. Journal of Neural Engineering. 14 (4), 045003 (2017).
- Sanders, J. I., Kepecs, A. A low-cost programmable pulse generator for physiology and behavior. Frontiers in Neuroengineering. 7, 43 (2014).
- Zimmermann, K., et al. Phenotyping sensory nerve endings in vitro in the mouse. Nature Protocols. 4 (2), 174-196 (2009).
- Dunham, J. P., Sales, A. C., Pickering, A. E. Ultrasound-guided, open-source microneurography: Approaches to improve recordings from peripheral nerves in man. Clinical Neurophysiology. 129 (11), 2475-2481 (2018).
- Levitt, H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 49 (2), 467 (1971).
- Turnquist, B., RichardWebster, B., Namer, B. Automated detection of latency tracks in microneurography recordings using track correlation. Journal of Neuroscience Methods. 262, 133-141 (2016).
- Kiernan, M. C., Burke, D., Andersen, K. V., Bostock, H. Multiple measures of axonal excitability: A new approach in clinical testing. Muscle Nerve. 23 (3), 399-409 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved