Method Article
Glioblastoma Relapse Post-Resection Model for Therapeutic Hydrogel Investigations
* These authors contributed equally
In This Article
Summary
The present protocol establishes a glioblastoma (GBM) relapse post-resection model using microscopy to investigate the therapeutic effect of an injectable, bioresponsive hydrogel in vivo.
Abstract
Tumor recurrence is an important factor indicative of a poor prognosis in glioblastoma (GBM). Many studies are attempting to identify effective therapeutic strategies to prevent the recurrence of GBM after surgery. Bioresponsive therapeutic hydrogels capable of sustaining locally released drugs are frequently used for the local treatment of GBM after surgery. However, research is limited due to the lack of a suitable GBM relapse post-resection model. Here, a GBM relapse post-resection model was developed and applied in therapeutic hydrogel investigations. This model was constructed based on the orthotopic intracranial GBM model, which is widely used in studies on GBM. Subtotal resection was performed on the orthotopic intracranial GBM model mouse to mimic the clinical treatment. The residual tumor was used to indicate the size of the tumor growth. This model is easy to build, can better mimic the situation of GBM surgical resection, and can be applied in various studies on the local treatment of GBM relapse post-resection. As a result, the GBM relapse post-resection model provides a unique GBM recurrence model for effective local treatment studies of relapse post-resection.
Introduction
Glioblastoma (GBM) is the most common malignant tumor among all central nervous system cancers1,2. Surgery is the first-line treatment for patients with GBM, and chemoradiation is the main adjuvant treatment following surgery. However, tumor recurrence often develops within 3-6 months in most GBM patients with various treatments3,4,5. Hence, there is an urgent need to develop more effective treatment strategies to prevent GBM recurrence.
Recent studies on GBM have mainly focused on primary tumors rather than recurrent tumors6. However, the most common problem that needs to be solved in the clinic is how to inhibit the recurrence of GBM after surgery. Therefore, research on the recurrence of GBM after surgery needs more attention. Bioresponsive therapeutic hydrogels are the most common vector used in studies on tumor recurrence after surgery7,8. However, due to the special structure of the central nervous system, it is difficult to develop a suitable GBM relapse post-resection model9, which is critical for the study of GBM recurrence.
This study has generated an improved GBM relapse post-resection model based on the orthotopic intracranial GBM model used in research on primary GBM. In this model, most of the tumors are removed by surgery with microscopy, and the residual tumor is detected by in vivo bioluminescent imaging and hematoxylin and eosin (H&E) staining. This model mimics the resection state of brain tumor patients and can be used in various studies on GBM relapse.
Protocol
All the animal experiments were approved and supervised by the Institutional Review Board and the Animal Ethics Committee of Nanjing Medical University (IACUC-1904004). C57BL/6J female mice, aged 6-8 weeks old, were used for the present study. The animals were obtained from a commercial source (see Table of Materials).
1. Animal preparation
- Weigh the mice, and anesthetize them with an intraperitoneal injection of 50 mg/kg pentobarbital sodium (see Table of Materials). Administer meloxicam (5mg/kg; i.p.) for perioperative analgesia. House the mice in a cage under a 12 h/12 h light/dark cycle, an ambient temperature of 24 °C, and a relative humidity of 50%.
NOTE: For the present study, the initial weight of the mice was about 22 g. - Remove the hair on the head of a mouse using a laboratory animal shaver, and fix the head and limbs on the stereotaxic apparatus (see Table of Materials).
NOTE: Sterilize all equipment before use. - Disinfect the head of the mouse with at least three alternating rounds of chlorhexidine or povidone-iodine scrub followed by alcohol, and cover the mouse with a sterile surgical drape. Apply ophthalmic ointment on both eyes to prevent drying.
- Cut the scalp of the mouse about 1 cm along the midline on the top of the right forehead with ophthalmic scissors.
NOTE: Confirm the surgical plane of anesthesia by the absence of pedal reflex before making the incision. - Tune the stereotaxic apparatus to ensure the herringbone seam and the front fontanel point are located at the same level.
2. Construction of the orthotopic intracranial GBM model
- Mark the point 1 mm to the front side of the front fontanel, 1.8 mm to the right side of the front fontanel, and 3 mm down from the front fontanel using a cotton swab dipped with gentian violet2 (see Table of Materials).
- Drill the point using a 1 mm diameter mini cranial drill (see Table of Materials) to create a pore size of about 1 mm diameter and 1 mm depth.
- Remove the exuded cerebrospinal fluid with a sterile cotton swab.
- Aspirate 5 µL of tumor cell suspension (GL261-Luci, 5 × 105 cells suspended in 5 µL of PBS) with a microsyringe.
NOTE: The GL261-Luci was obtained from a commercial source (see Table of Materials). - Align the needle tip of the microsyringe vertically with the skull drilling hole, insert until the needle tip enters the skull plane for 3 mm, and return the needle by 0.5 mm7.
- Open the microsyringe, and inject with a speed of 1 µL/min.
- Retain the needle for 10 min after injection.
- Withdraw the microsyringe slowly, and press the injection point with a sterile dry cotton ball.
- Suture the scalp with a nonabsorbable surgical suture (10-0, see Table of Materials), and disinfect the incision again.
- Monitor the animal's health, and keep it in warm conditions.
- Move the mouse back to the housing cage after the mouse wakes up.
- Image the mouse with an in vivo bioluminescent imaging system (see Table of Materials) to detect the transplanted tumor on the 10th day after tumor-bearing.
NOTE: Luciferase loaded in the GBM cells, GL261, was used for the bioluminescent imaging.- Anesthetize the mouse with 1.5% isoflurane with an oxygen flow rate of 0.6L/min. After confirming anesthesia depth via lack of pedal reflex, inject the mouse intraperitoneally with potassium fluorescein (10 mg/mL, see Table of Materials), and 11 s later, perform the in vivo bioluminescent imaging. When the fluorescence value reaches ~5 × 105, the procedure is successful.
NOTE: Anesthetize the animal before imaging. Maintain anesthesia via nose cone supplied with vaporized isoflurane.
- Anesthetize the mouse with 1.5% isoflurane with an oxygen flow rate of 0.6L/min. After confirming anesthesia depth via lack of pedal reflex, inject the mouse intraperitoneally with potassium fluorescein (10 mg/mL, see Table of Materials), and 11 s later, perform the in vivo bioluminescent imaging. When the fluorescence value reaches ~5 × 105, the procedure is successful.
- Select the mice with successful tumor-bearing to construct the GBM relapse post-resection model.
3. Construction of the GBM relapse post-resection model
- When the tumor size in the orthotopic intracranial GBM model mice becomes ~6.5 × 105, select the mice for the postoperative relapse model.
- Weigh the orthotopic intracranial GBM model mice, and anesthetize the mice with an intraperitoneal injection of 50 mg/kg pentobarbital sodium.Administer meloxicam (5mg/kg; i.p.) for perioperative analgesia.
- Repeat the process of steps 1.2−1.5.
- Separate the scalp tissue and skull, and check the drilling hole used to build the orthotopic intracranial GBM model. If the hole has healed, identify the hole using stereotactic apparatus, and follow the procedure mentioned in steps 2.1-2.3.
- Expand the hole diameter to 5 mm with a skull drill, and remove the exuded cerebrospinal fluid with a sterile cotton swab.
- Focus the microscope on the head of the mouse, and adjust the settings to ensure the drilling hole is located in the center of the field of vision.
- Cut the meninges with microscissors, and remove part of the tumor tissue with a micro curette and a micro scalpel under the microscope.
NOTE: Regarding the meninges, when the skull is opened, the thin membrane close to the brain tissue is the meninges. - Stop the bleeding with sterile gauze, and wash the incision with sterile physiological saline.
- Inject the commercially available hydrogel (10 µL, see Table of Materials) into the resection cavity with a 1 mL syringe, suture the scalp with a nonabsorbable surgical suture (10-0), and disinfect the incision again (Figure 1).
- Monitor the animal's health, and keep the mouse warm.
- Move the mouse back to the housing cage after the mouse wakes up.
- Perform in vivo bioluminescent imaging to detect the transplanted tumor 1 day later to quantify the size of the residual tumor (Figure 2 and Figure 3).
- Weigh the mice every 2 days until the terminal endpoint.
NOTE: For the present study, the terminal endpoint was day 30, and the mice were euthanized by an overdose of pentobarbital sodium with an intraperitoneal injection.
Results
The process of construction of the GBM relapse post-resection model is shown in Figure 1. The resection cavity after the tumor was partially removed under the microscopy is shown. The hydrogel was injected into the resection cavity with a syringe to demonstrate the therapeutic effect. The schedule of the experimental design is shown in Figure 2A. After the GBM cells were implanted into the brain of the mice, the tumor growth was tested by in vivo bioluminescent imaging on day 10. The resection was performed on day 11, and the hydrogel was then injected into the resection cavity. The in vivo bioluminescent imaging testing was performed on day 15, day 20, day 25, and day 30 to monitor the residual tumor growth. As shown in Figure 2B,C, the size of the tumors in the GBM relapse post-resection model was significantly smaller than those in the orthotopic intracranial GBM model, as shown by the in vivo bioluminescent imaging testing. On day 25, the tumors recurred significantly post-resection (Figure 2D). The H&E staining confirmed that the GBM relapse post-resection model was constructed successfully and that residual tumors significantly recurred after the resection (Figure 3A,B).
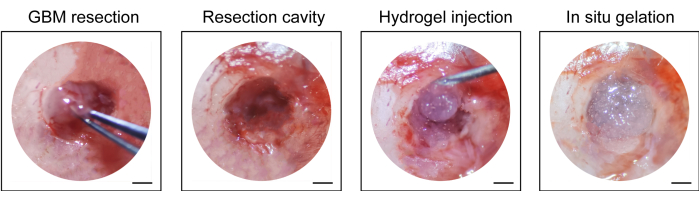
Figure 1: Intraoperative view of the tumor resection and injection of the hydrogel. Part of the tumor tissue was removed with a micro curette and micro scalpel under the microscope, and hydrogel was injected into the resection cavity. Scale bars: 50 µm. This figure has been modified from Sun et al.10. Please click here to view a larger version of this figure.
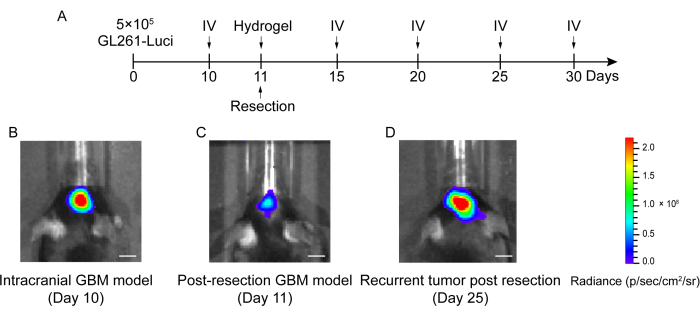
Figure 2: Schedule of the experimental design and the in vivo bioluminescent imaging of mice in the intracranial and post-resection GBM model. (A) The schedule of the experimental design showing that the resection was performed on day 11 and that the in vivo bioluminescent imaging (IV) was performed on day 10, day 15, day 20, day 25, and day 30. (B) The intracranial GBM model mice showed a large tumor size on day 10, (C) the tumor size was significantly reduced after resection on day 11, and (D) the tumor size increased after resection on day 25 in the post-resection GBM model. The control group included the GBM relapse post-resection model mice with no treatment. A total of 42 mice were used in this study. Scale bars: 100 µm. This figure has been modified from Sun et al.10. Please click here to view a larger version of this figure.
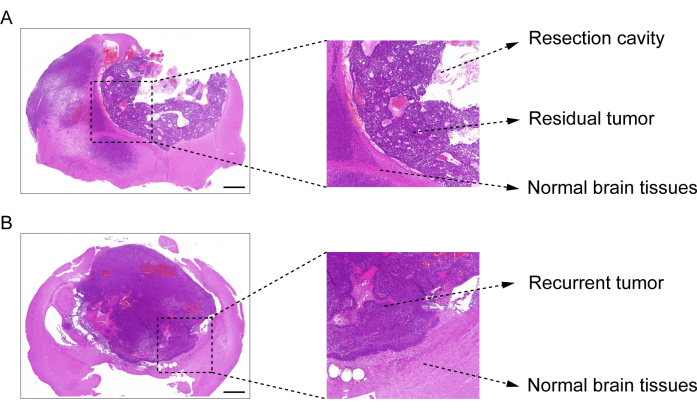
Figure 3: H&E staining image of brain tissue from the post-resection GBM model. (A) An H&E staining image demonstrating the resection cavity, residual tumor, and normal brain tissue. The brain tissue was collected 1 day after the resection. (B) An H&E staining image demonstrating the recurrent tumor and the normal brain tissue. The brain tissue was collected on day 12 after the resection. Scale bars: 100 µm. This figure has been modified from Sun et al.10. Please click here to view a larger version of this figure.
Discussion
Surgery remains the first choice for most GBM patients11. Due to the characteristic of invasive growth of GBM, a small number of tumor cells still remain after micro neurosurgical techniques, resulting in eventual tumor recurrence12. How to inhibit the recurrence of GBM after surgery has become the focus of GBM-related research. However, due to the complex anatomical structure of brain tissue, the construction of a suitable postoperative GBM model has become the primary problem to be solved in this field.
This study developed a GBM relapse post-resection model. In the process of constructing this model, the construction of the orthotopic intracranial GBM model is critical. After this model has been developed successfully, the resection needs to be performed at the right time. The recommended time is when the fluorescence value of the tumor size is about 6.5 × 105. To reduce the mortality of the mice, resection under anesthesia was performed with 40 mg/kg 1% pentobarbital sodium by intraperitoneal injection. However, the resection was difficult to perform, and the mice often moved due to the small dose of the anesthetic. On this basis, the dose of the anesthetic was increased to 50 mg/kg. After increasing the anesthetic dose, the intraoperative responses of the mice disappeared, and the resection was performed successfully. Isoflurane gas can also be used in this protocol.
In this study, GL261-Luci cells were used to develop the model; therefore, more GBM cell lines must be used to validate the protocol in the future. To make the protocol more convincing, various GBM mouse models, such as genetically engineered GBM mouse models, need to be used. In addition, MRI may be the best means to detect the recurrence of tumors after surgery.
In summary, in this work, a GBM relapse post-resection model has been developed. In this model, tumor recurrence is monitored by assessing the residual tumor's growth after resection. Although this model cannot be considered to completely mimic tumor recurrence, the resection style in this model is similar to the standard of maximally safe surgery in the clinical treatment of GBM patients. This work provides a convenient and feasible method of constructing the GBM relapse post-resection model and represents an advancement in the field of research on GBM relapse post-resection.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by project grants from The National Natural Science Foundation of China (82071767 and 82171781).
Materials
| Name | Company | Catalog Number | Comments |
| Gentian violet | Sigma | C6158 | |
| GL261-Luci | Shanghai Zhong Qiao Xin Zhou Biotechnology Co.,Ltd. | ZQ0932 | |
| In vivo bioluminescent imaging system | Tanon | Tanon ABL X6 | |
| Laboratory animal shaver | Beyotime Biotechnology | FS600 | |
| Mice | Beijing Vital River Laboratory Animal Technology Co., Ltd. | ||
| Micro curette | Belevor Medical Co.,Ltd. | ||
| Micro scalpel | Belevor Medical Co.,Ltd. | ||
| Microscope | Shanghai Xiangfan Instrument Co., Ltd | JSZ5A/B | |
| Microsyringe | Hamilton | 87943 | |
| Mini cranial drill | RWD | 78001 | |
| Nonabsorbable surgical suture | Shanghai Yuyan Instruments Co.,Ltd. | ||
| Pentobarbital sodium | ChemSrc | 57-33-0 | |
| PVA-TSPBA hydrogel | Aladdin | 9002-89-5 | |
| Stereotaxic apparatus | RWD | 68043 |
References
- Lim, M., Xia, Y., Bettegowda, C., Weller, M. Current state of immunotherapy for glioblastoma. Nature Reviews Clinical Oncology. 15 (7), 422-442 (2018).
- Wang, X., et al. In situ targeting nanoparticles-hydrogel hybrid system for combined chemo-immunotherapy of glioma. Journal of Controlled Release. 345, 786-797 (2022).
- Binder, Z. A., O'Rourke, D. M. Glioblastoma: The current state of biology and therapeutic strategies. Cancer Research. 82 (5), 769-772 (2022).
- Kauer, T. M., Figueiredo, J. L., Hingtgen, S., Shah, K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nature Neuroscience. 15 (2), 197-204 (2011).
- Jiang, X., et al. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proceedings of the National Academy of Sciences of the United States of America. 113 (48), 13857-13862 (2016).
- Quail, D. F., Joyce, J. A. The microenvironmental landscape of brain tumors. Cancer Cell. 31 (3), 326-341 (2017).
- Zhang, J., et al. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nature Nanotechnology. 16 (5), 538-548 (2021).
- Ruan, H., et al. A dual-bioresponsive drug-delivery depot for combination of epigenetic modulation and immune checkpoint blockade. Advanced Materials. 31 (17), 1806957 (2019).
- Louveau, A., et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 523 (7560), 337-341 (2015).
- Sun, S., et al. Immunostimulant in situ hydrogel improves synergetic radioimmunotherapy of malignant glioblastoma relapse post-resection. Advanced Functional Materials. 32 (43), 2205038 (2022).
- Liu, D. K., Sulman, E. P., Wen, P. Y., Kurz, S. C. Novel therapies for glioblastoma. Current Neurology and Neuroscience Reports. 20 (7), 19 (2020).
- Mellinghoff, I. K., Cloughesy, T. F. Balancing risk and efficiency in drug development for rare and challenging tumors: A new paradigm for glioma. Journal of Clinical Oncology. 40 (30), 3510-3519 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved