A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Hand-Rearing Method for Infant Marmosets
In This Article
Summary
Here, we describe a hand-rearing method for raising infant marmosets in an animal incubator. This method greatly increases the survival rate of marmoset infants, which provides the opportunity to study the development of marmoset infants with similar genetic backgrounds raised in different postnatal environments.
Abstract
The common marmoset (Callithrix jacchus) is a small and highly social New World monkey with high reproduction rates, which has been proven to be a compelling non-human primate model for biomedical and neuroscience research. Some females give birth to triplets; however, the parents cannot raise all of them. To save these infants, we have developed a hand-rearing method for raising newborn marmosets. In this protocol, we describe the formula of the food, the time for feeding, the configuration of the temperature and humidity, as well as the adaptation of the hand-reared infants to the colony environment. This hand-rearing method significantly increases the survival rate of marmoset infants (without hand-rearing: 45%; with hand-rearing: 86%) and provides the opportunity to study the development of marmoset infants with similar genetic backgrounds raised in different postnatal environments. As the method is practical and easy to use, we anticipate that it could also be applied to other labs working with common marmosets.
Introduction
The common marmoset (Callithrix jacchus) is a small and arboreal New World monkey originating from South and Central America. The use of marmosets in biomedical research has grown rapidly over the past decades due to several key advantages of marmosets compared with other non-human primates (NHPs), including their smaller body size, easier handling and breeding in captivity, shorter gestation time, earlier sexual maturation, and lower zoonotic risks1,2,3,4,5,6. The common marmoset has a similar brain structure and brain function to humans and displays a rich repertoire of vocalizations and highly social behavior with rich emotions. It is a compelling NHP model for different types of neuroscience studies, such as studies on sensory processing7,8,9,10,11,12,13,14, vocal communication15,16,17,18,19, models of spinal cord injury20,21,22,23, Parkinson's disease24,25,26,27,28, and age-related diseases29. Compared with other NHPs, the common marmoset has a relatively high reproduction rate, which is potentially useful for transgenic modification30,31,32. This primate is also widely used in pharmacology, angiography, and pathogen and immune studies33,34,35,36,37,38,39. However, the supply of marmosets remains very limited, especially in China, and cannot meet the rapidly growing needs of scientific research.
In marmoset colonies, the adult animals are fed once or twice per day, and a few institutions alter the diet for juvenile marmosets40. Generally, infant marmosets usually grasp firmly onto the body of the father or elder siblings for daily care and are handed to the mother several times per day for milk. Some female marmosets give birth to triplets, and in this case, one or two infants cannot survive due to a lack of milk; moreover, some parents do not take care of their infants because they lack nursing experience or for other unknown reasons. This is a big loss for many laboratories. A few studies have reported methods of nutrition management for adult marmosets in captive settings40,41,42 utilizing foods and formulas with different macronutrient compositions, vitamins, and minerals, as well as different feeding protocols for enrichment (mashed, gelled, purified, or canned)2,41. One previous study reported a collaborative rearing method for marmoset triplets43, in which caregivers take one infant per day, hand-feed it throughout the day, and exchange it for another of the triplets on the next day. Although this method allows the infants to have parental care, it requires an experienced caregiver to grab the infant from the body of the parents every day and is labor-intensive. So far, no study has reported a detailed, step-by-step hand-rearing method for newborn marmosets.
The goal of the current study is to provide a hand-rearing method for those interested in marmoset development but with limited resources. In contrast to the previous collaborative rearing method43, the current method is an alternative that causes less disturbance to the infant's family and is easy to learn. Based on the basic rules of breastfeeding and 5 years of practice, this paper describes a hand-rearing method for raising infant marmosets that includes the preparation of the food, a timetable for feeding, the configuration of the temperature and humidity of the animal incubator, as well as the adaptation of the infant animals to the colony environment.
Protocol
All the experimental procedures were approved by the Animal Use and Care Committee of Zhejiang University and followed the National Institutes of Health (NIH) guidelines.
1. Housing and husbandry44
- Set the colony room with a 12 h:12 h day/night cycle, the temperature at 26-28 °C, and the relative humidity at 45%-55%.
- Pair male and female marmosets at 2-6 years old, and hold them in cages (850 mm x 800 mm x 800 mm) with sufficient space and fresh air with a 24 h ventilation system.
- Provide resting boards, swings, perches, and hammocks in the cages.
- Feed the pair of marmosets with fresh water and 30-40 g of food twice a day, including cereal, eggs, sweet potatoes, honey, fruit, vegetables, and mealworms.
NOTE: Veterinarians and experimenters must inspect the animal facility at least once per day to ensure that any sick individuals are diagnosed and treated immediately.
2. Preparation before the birth of the marmoset infants
- Care for the pregnant marmosets
NOTE: The time of conception was diagnosed by palpation (typically 10-20 days after the start of the embryonic period), and we also referred to the reproductive history of the animal subjects.- Provide a larger space and minimal human disturbance for the breeding pairs.
- Feed the breeding pairs with additional food such as mealworms, eggs, yogurt, and dried fruit to guarantee nutrition for the females.
- Take care of and frequently check the pregnant marmosets to prepare for their parturition.
NOTE: The gestation period of the marmoset is estimated to be 148 days ± 4.3 days45.
- Prepare the following items: an animal incubator (855 mm [W] x 470 mm [L] x 440 mm [H]), disposable diaper pads (M/L sizes), baby wipes, plush toys (Figure 1A), toy rollers (Figure 1B), climbing frames (Figure 1B), blankets (10 cm х 10 cm, Figure 1C), and an electronic scale (precision of 0.2 g, Figure 1F).
NOTE: To avoid animal hanging, the plush toys should not have loop structures. - Food and feeding appliance preparation
- Prepare the following items: baby formula (suitable for 0-12 months of age), baby rice paste (suitable for 0-6 months of age), electric water kettle, beaker (100 mL), heating pad, plastic weighing dish (80 mm x 80 mm x 22 mm, Figure 1D), sterile centrifuge tubes (50 mL), disposable sterile syringes (1-5 mL), intravenous injectors (for custom-made feeding nipples) (Figure 1E), and swabs (80-100 cm).
NOTE: To make a nipple for feeding, a cut is made 1 cm from the end of an intravenous injector attached to a syringe (Figure 1E).
- Prepare the following items: baby formula (suitable for 0-12 months of age), baby rice paste (suitable for 0-6 months of age), electric water kettle, beaker (100 mL), heating pad, plastic weighing dish (80 mm x 80 mm x 22 mm, Figure 1D), sterile centrifuge tubes (50 mL), disposable sterile syringes (1-5 mL), intravenous injectors (for custom-made feeding nipples) (Figure 1E), and swabs (80-100 cm).
- Recording form preparation
- Prepare a form, usually several pages long, for each infant marmoset to record basic information such as the name, birth date, birth weight, parents, other basic information of interest such as the head circumference and tail length, breeding information such as the breeding date and time, the amount (mL) of the food intake, the defecation state (yes/no, hard/loose), and the incubator temperature and humidity.
NOTE: Usually, the body weight is measured and recorded twice a day, once before the first meal and once before the last meal.
- Prepare a form, usually several pages long, for each infant marmoset to record basic information such as the name, birth date, birth weight, parents, other basic information of interest such as the head circumference and tail length, breeding information such as the breeding date and time, the amount (mL) of the food intake, the defecation state (yes/no, hard/loose), and the incubator temperature and humidity.
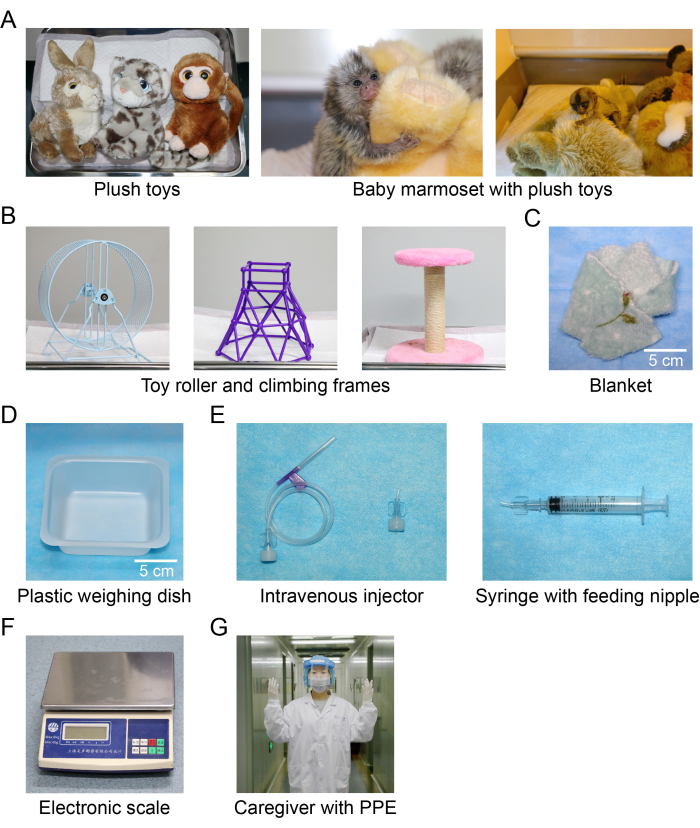
Figure 1: Photos of the items in the incubator and the feeding tools and accessories. (A) Plush toys; (B) toy roller and climbing frames; (C) blanket; (D) plastic weighing dish; (E) intravenous injector and syringe with a custom-made feeding nipple; (F) electronic scale; (G) caregiver with personal protective equipment. Please click here to view a larger version of this figure.
3. Hand-rearing procedure
- Clean and sterilize the room ahead of the due date.
- Spray hypochlorous acid or 75% ethyl alcohol on the floor and table, leave for 30 s, and then wipe the table, and mop the floor.
- Set the temperature of the incubator at 35 °C and the humidity at 40%. Usually, to simulate the basic temperature requirement of infant marmosets, as Table 1 shows, before postnatal day 14 (P14), keep the incubator temperature at 35 °C, and from P15 on, lower the temperature by 0.5 °C every 3 days. Keep the humidity at 40%-45% inside the incubator, which is close to the colony humidity and keeps the fur of the infants dry.
- Tile a disposable diaper pad to cover the chassis of the incubator.
- To minimize the stress of the infants, put a couple of blankets and plush toys in the incubator ahead of introducing the infant marmosets, which tend to mimic the adult marmosets.
NOTE: The blankets and plush toys are put in the home cage of the breeding pairs for 1 day before use. - Put the infant marmosets into the incubator and place them on the plush toys once they are separated from their parents in the home cage.
NOTE: To avoid social isolation and in accord with animal welfare, generally, two infants are chosen together for hand-rearing.- Wear sterilized personal protective equipment (PPE, Figure 1G) before feeding.
- Warm a couple of blankets to 35 °C.
- Gently hold the infant marmoset with warm blankets, and obtain the weight of the animal as the birth weight.
- Transfer the infant marmoset into the incubator with warm blankets.
- Take records, as mentioned in step 2.4.
- Blend food ingredients and feed the infant marmoset.
- Dissolve 5 g of baby formula in 30 mL of 50 °C boiled water in a 50 mL sterile centrifuge tube.
NOTE: Infant marmosets at different postnatal ages need different food recipes. Table 2 includes the different dosages of baby formula, rice paste, and water at postnatal ages from P1 to P60. The dosage is usually enough for 1 day. Blend the ingredients before the first meal, and store the rest of the food in a 4 °C fridge. Heat the food to 30-35 °C for each meal. - Take 1 mL of food with a 1 mL syringe, and lid the syringe with a custom-made feeding nipple.
NOTE: Select a syringe of the proper size, referring to Table 2. - Keep the food temperature at 30-35 °C with the heating pad.
- Warm the hands before feeding, and gently hold the infant marmoset with a warm blanket in one hand in the incubator.
- Put the feeding nipple into the mouth of the infant marmoset while the head of the infant marmoset is softly held with the thumb and index finger of the holding hand of the caregiver, and slowly push the food out of the syringe at a constant velocity.
NOTE: Never push the food faster than the infant marmoset swallows. Pushing fast can cause choking, with food coming out from the nose through the throat. This can cause diseases such as pneumonia, which can even lead to death. If the food overflows, the infant marmoset will struggle. Whenever this occurs, stop feeding and wipe the food off the animal's face carefully. Continue to feed after the marmoset starts behaving normally.
- Dissolve 5 g of baby formula in 30 mL of 50 °C boiled water in a 50 mL sterile centrifuge tube.
- After the infant marmoset consumes a proper amount of food, wipe its anus with a swab with warm water, which both cleans the anus and promotes defecation.
- Observe the animal for a few minutes, and check the locomotion and defecation of the animal.
- Record the feeding time, the amount (mL) of the food intake, the defecation state (yes/no, hard/loose), and the incubator temperature and humidity.
NOTE: Wrap the infant marmoset with warm blankets during weighing to avoid the infant getting cold or injured. - Keep the chassis of the incubator clean by picking up solid feces or changing to a new disposable diaper pad.
- Before P50, feed the infant marmoset following steps 3.3-3.7, and use the dosage of food ingredients and the feeding time and frequency shown in Table 2.
- From P50 on, the infant marmoset is usually ready for voluntary eating.
- Use plastic weighing dishes instead of syringes. Prepare the food by mixing food ingredients directly in the dish; refer to Table 2 for the amounts.
- Put the food dish in the incubator, and fix the bottom in case it is turned over. Observe for a few minutes to make sure the infant marmosets eat the food. For the first few times, guide the animal to eat voluntarily by luring the animal to the food dish and guiding its mouth to touch the food several times.
NOTE: Never let the animal's nose touch the food. Usually, the animal learns to voluntarily eat in 1 day.
4. Acclimation before the return of the infant marmosets to the colony
NOTE: Usually, the hand-rearing is finished when the infant marmosets learn to eat by themselves. There are a few adaptation procedures to be carried out before they are returned to the home cage in the marmoset colony.
- Move the infant marmosets from the animal incubator to small cages (45 cm x 45 cm x 40 cm), which are similar to a birdcage. Hang a water bottle (50 mL) on each small cage.
- Transfer the small cages with the infant marmosets to the colony, and position them close to the family cage.
- Feed the infant marmosets separately with a plastic weighing dish for 1 week, mixing the food according to the daily recipes prepared for the whole colony.
- Make a record of the body weight and defecation status once per day.
5. Infant marmosets returning to the family cage
NOTE: After living in the small cage for 7-10 days, the infant marmosets usually adapt to the colony environment well and exhibit no more anxiety.
- Put the infant marmosets back into the family cage in the morning.
- Observe the animals for at least 15 min to make sure there is no biting, fighting, or chasing between the family members and the new entrants.
NOTE: If biting, fighting, or chasing occurs, separate the infant from the others as soon as possible; and try to return the infant to its family one more time on another day. If the failure occurs again, select another family to foster it. The family groups that have rich parenting experience are the first choice for fostering. Put a plush toy in a new cage to accompany the infant monkey if no family accepts it. - Stop feeding the infant marmosets separately, and start feeding them using the diet for the colony.
- Pay close attention to the activity of the infant marmosets for 1 week after they return to the family cage.
- Measure the body weights of the infant marmosets every 2 days, and make a record. If they lose weight, feed them extra nutritional food by syringe in the family cage.
- Provide daily care for the infant marmosets, as for the marmosets in the colony.
Results
Body weight is a key index of animal body development and is used as an indicator of the health status of the marmosets in this protocol. In this work, the body weights of the hand-reared animals increased gradually with age (Figure 2A, n = 16), similar to the weights of newborn infants in a previous study46. To minimize the disturbance to the breeding families in the colony, we did not weigh the infant marmosets in the colony every day. We obtained the weights of the...
Discussion
The common marmoset is a very useful NHP model for biomedical and neuroscience research. However, marmoset resources are too limited to meet the rapidly growing needs. In this work, we have developed a hand-rearing method that not only increases the survival rate of marmoset infants but also provides an opportunity to study their postnatal development. This hand-rearing method is practical and easy to learn and is, therefore, easily applicable to other labs working with common marmosets.
Some ...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors would like to thank Mingxuan Li for his editing of the grammar and polishing of the early version of this manuscript. This work was supported by the Zhejiang Province Natural Science Foundation of China (LD22H090003); the Natural Science Foundation of China (32170991 and 32071097), STI2030-Major Projects 2021ZD0204100 (2021ZD0204101) and 2022ZD0205000 (2022ZD0205003); and the MOE Frontier Science Center for Brain Science & Brain-Machine Integration, Zhejiang University.
Materials
| Name | Company | Catalog Number | Comments |
| animal incubator | RCOM, Korea | MX - BL600N, 855 mm (W) x 470 mm (L) x 440 mm (H) | |
| baby milk powder | Meadjohnson, America | suitable for 0-12 months of age, executive standard - GB25596 | |
| baby rice paste | HEINZ, China | suitable for 0-6 months of age, executive standard - GB10769 | |
| baby wipes | babycare, China | soft | |
| beaker | ShuNiu, China | 100 mL | |
| blankets | Grace, China | 10 cm × 10 cm, soft | |
| climbing frame | WowWee, China | firm and no small circular structures | |
| disposable diaper pads | Hi Health Pet, China | either M or L size | |
| disposable sterile syringe | Cofoe, China | 1 mL, 2.5 mL, 3 mL, 5 mL, 10 mL | |
| electronic scale | YouSheng, China | measuring range from 0 to 6,000 g with precision of 0.2 g | |
| intravenous injector | HD, China | 0.55 mm x 20 mm needle | |
| kettle | FGA, China | warm-keeping kettle 1,500 mL | |
| lactulose | BELCOL, China | to solve constipation | |
| plastic weighing dish | SKSLAB, China | 80 mm x 80 mm x 22 mm, used as a bowl | |
| plush toy | Lebiyou, China | soft | |
| probiotic powder | G-Pet, China | to regulate gastrointestinal environment | |
| sterile centrifuge tube | NEST, China | 50 mL | |
| swab | OYEAH, China | 80 - 100 mm | |
| toy roller | WowWee, China | firm and no small circular structures |
References
- Miller, C. T., et al. Marmosets: A neuroscientific model of human social behavior. Neuron. 90 (2), 219-233 (2016).
- Ross, C. N., Colman, R., Power, M., Tardif, S. Marmoset metabolism, nutrition, and obesity. ILAR Journal. 61 (2-3), 179-187 (2020).
- Kishi, N., Sato, K., Sasaki, E., Okano, H. Common marmoset as a new model animal for neuroscience research and genome editing technology. Development, Growth & Differentiation. 56 (1), 53-62 (2014).
- Prins, N. W., et al. Common marmoset (Callithrix jacchus) as a primate model for behavioral neuroscience studies. Journal of Neuroscience Methods. 284, 35-46 (2017).
- Tokuno, H., Watson, C., Roberts, A., Sasaki, E., Okano, H. Marmoset neuroscience. Neuroscience Research. 93, 1-2 (2015).
- Hodges, J. K., Henderson, C., Hearn, J. P. Relationship between ovarian and placental steroid production during early pregnancy in the marmoset monkey (Callithrix jacchus). Journal of Reproduction and Fertility. 69 (2), 613-621 (1983).
- Troilo, D., Judge, S. J. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus). Vision Research. 33 (10), 1311-1324 (1993).
- Mitchell, J. F., Leopold, D. A. The marmoset monkey as a model for visual neuroscience. Neuroscience Research. 93, 20-46 (2015).
- Hung, C. C., et al. Functional MRI of visual responses in the awake, behaving marmoset. NeuroImage. 120, 1-11 (2015).
- Gao, L., Kostlan, K., Wang, Y., Wang, X. Distinct subthreshold mechanisms underlying rate-coding principles in primate auditory cortex. Neuron. 91 (4), 905-919 (2016).
- Gao, L., Wang, X. Subthreshold activity underlying the diversity and selectivity of the primary auditory cortex studied by intracellular recordings in awake marmosets. Cerebral Cortex. 29 (3), 994-1005 (2019).
- Gao, L., Wang, X. Intracellular neuronal recording in awake nonhuman primates. Nature Protocols. 15, 3615-3631 (2020).
- Wang, X., et al. Corticofugal modulation of temporal and rate representations in the inferior colliculus of the awake marmoset. Cerebral Cortex. 32 (18), 4080-4097 (2022).
- Wang, X., et al. Selective corticofugal modulation on sound processing in auditory thalamus of awake marmosets. Cerebral Cortex. 33 (7), 3372-3386 (2022).
- Kajikawa, Y., et al. Coding of FM sweep trains and twitter calls in area CM of marmoset auditory cortex. Hearing Research. 239 (1-2), 107-125 (2008).
- Choi, D., Bruderer, A. G., Werker, J. F., et al. Sensorimotor influences on speech perception in pre-babbling infants: Replication and extension of Bruderer et al. Psychonomic Bulletin & Review. 26 (4), 1388-1399 (2019).
- Eliades, S. J., Miller, C. T. Marmoset vocal communication: Behavior and neurobiology. Developmental Neurobiology. 77 (3), 286-299 (2017).
- Roy, S., Zhao, L., Wang, X. Distinct neural activities in premotor cortex during natural vocal behaviors in a New World primate, the common marmoset (Callithrix jacchus). Journal of Neuroscience. 36 (48), 12168-12179 (2016).
- Simões, C. S., et al. Activation of frontal neocortical areas by vocal production in marmosets. Frontiers in Integrative Neuroscience. 4, 123 (2010).
- Iwanami, A., et al. Transplantation of human neural stem cells for spinal cord injury in primates. Journal of Neuroscience Research. 80 (2), 182-190 (2005).
- Schorscher-Petcu, A., Dupré, A., Tribollet, E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neuroscience Letters. 461 (3), 217-222 (2009).
- Bowes, C., Burish, M., Cerkevich, C., Kaas, J. Patterns of cortical reorganization in the adult marmoset after a cervical spinal cord injury. Journal of Comparative Neurology. 521 (15), 3451-3463 (2013).
- Kondo, T., et al. Histological and electrophysiological analysis of the corticospinal pathway to forelimb motoneurons in common marmosets. Neuroscience Research. 98, 35-44 (2015).
- Nash, J. E., et al. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson's disease. Experimental Neurology. 165 (1), 136-142 (2000).
- van Vliet, S. A., et al. Neuroprotective effects of modafinil in a marmoset Parkinson model: Behavioral and neurochemical aspects. Behavioural Pharmacology. 17 (5-6), 453-462 (2006).
- van Vliet, S. A., Vanwersch, R. A., Jongsma, M. J., Olivier, B., Philippens, I. H. Therapeutic effects of Delta9-THC and modafinil in a marmoset Parkinson model. European Neuropsychopharmacology. 18 (5), 383-389 (2008).
- Philippens, I. H., t Hart, B. A., Torres, G. The MPTP marmoset model of parkinsonism: a multi-purpose non-human primate model for neurodegenerative diseases. Drug Discovery Today. 15 (23-24), 985-990 (2010).
- Santana, M. B., et al. Spinal cord stimulation alleviates motor deficits in a primate model of Parkinson disease. Neuron. 84 (4), 716-722 (2014).
- Tardif, S. D., Mansfield, K. G., Ratnam, R., Ross, C. N., Ziegler, T. E. The marmoset as a model of aging and age-related diseases. ILAR Journal. 52 (1), 54-65 (2011).
- Sasaki, E., et al. Generation of transgenic non-human primates with germline transmission. Nature. 459, 523-527 (2009).
- Sasaki, E. Prospects for genetically modified non-human primate models, including the common marmoset. Neuroscience Research. 93, 110-115 (2015).
- Park, J. E., Sasaki, E. Assisted reproductive techniques and genetic manipulation in the common marmoset. ILAR Journal. 61 (2-3), 286-303 (2020).
- Smith, D., Trennery, P., Farningham, D., Klapwijk, J. The selection of marmoset monkeys (Callithrix jacchus) in pharmaceutical toxicology. Laboratory Animals. 35 (2), 117-130 (2001).
- Smith, T. E., Tomlinson, A. J., Mlotkiewicz, J. A., Abbott, D. H. Female marmoset monkeys (Callithrix jacchus) can be identified from the chemical composition of their scent marks. Chemical Senses. 26 (5), 449-458 (2001).
- Jagessar, S. A., et al. Induction of progressive demyelinating autoimmune encephalomyelitis in common marmoset monkeys using MOG34-56 peptide in incomplete freund adjuvant. Journal of Neuropathology and Experimental Neurology. 69 (4), 372-385 (2010).
- Kap, Y. S., Laman, J. D., 't Hart, B. A. Experimental autoimmune encephalomyelitis in the common marmoset, a bridge between rodent EAE and multiple sclerosis for immunotherapy development. Journal of Neuroimmune Pharmacology. 5 (2), 220-230 (2010).
- Carrion, R., Patterson, J. L. An animal model that reflects human disease: The common marmoset (Callithrix jacchus). Current Opinion in Virology. 2 (3), 357-362 (2012).
- Jagessar, S. A., et al. Overview of models, methods, and reagents developed for translational autoimmunity research in the common marmoset (Callithrix jacchus). Experimental Animals. 62 (3), 159-171 (2013).
- Feng, Z., et al. Biologically excretable aggregation-induced emission dots for visualizing through the marmosets intravitally: Horizons in future clinical nanomedicine. Advanced Materials. 33 (17), 2008123 (2021).
- Goodroe, A., et al. Current practices in nutrition management and disease incidence of common marmosets (Callithrix jacchus). Journal of Medical Primatology. 50 (3), 164-175 (2021).
- Power, M. L., Koutsos, L., Marini, R., Wachtman, L., Tardif, S., Mansfield, K., Fox, J. Chapter 4 - Marmoset nutrition and dietary husbandry. The Common Marmoset in Captivity and Biomedical Research. , 63-76 (2019).
- Gore, M. A., et al. Callitrichid nutrition and food sensitivity. Journal of Medical Primatology. 30 (3), 179-184 (2001).
- Hearn, J. P., Burden, F. J. Collaborative' rearing of marmoset triplets. Laboratory Animals. 13 (2), 131-133 (1979).
- Cao, X., et al. Effect of a high estrogen level in early pregnancy on the development and behavior of marmoset offspring. ACS Omega. 7 (41), 36175-36183 (2022).
- Watakabe, A., et al. Application of viral vectors to the study of neural connectivities and neural circuits in the marmoset brain. Developmental Neurobiology. 77 (3), 354-372 (2017).
- Takahashi, D. Y., et al. The developmental dynamics of marmoset monkey vocal production. Science. 349 (6249), 734-738 (2015).
- Malukiewicz, J., et al. The gut microbiome of exudivorous marmosets in the wild and captivity. Scientific Reports. 12 (1), 5049 (2022).
- Shigeno, Y., et al. Comparison of gut microbiota composition between laboratory-bred marmosets (Callithrix jacchus) with chronic diarrhea and healthy animals using terminal restriction fragment length polymorphism analysis. Microbiology and Immunology. 62 (11), 702-710 (2018).
- Baxter, V. K., et al. Serum albumin and body weight as biomarkers for the antemortem identification of bone and gastrointestinal disease in the common marmoset. PLoS One. 8 (12), e82747 (2013).
- Tardif, S. D., et al. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus). Obesity. 17 (8), 1499-1505 (2009).
- Wachtman, L. M., et al. Differential contribution of dietary fat and monosaccharide to metabolic syndrome in the common marmoset (Callithrix jacchus). Obesity. 19 (6), 1145-1156 (2011).
- Power, M. L., Ross, C. N., Schulkin, J., Ziegler, T. E., Tardif, S. D. Metabolic consequences of the early onset of obesity in common marmoset monkeys. Obesity. 21 (12), E592-E598 (2013).
- Shimizu, K., et al. Peer-social response in 4 juvenile marmosets represented the emotional development traits depending on family structure. Neuroscience Research. 65, S244 (2009).
- Schultz-Darken, N., Braun, K. M., Emborg, M. E. Neurobehavioral development of common marmoset monkeys. Developmental Psychobiology. 58 (2), 141-158 (2016).
- Gultekin, Y. B., Hage, S. R. Limiting parental feedback disrupts vocal development in marmoset monkeys. Nature Communications. 8, 14046 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved