A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Hepatic Glucose Production, Ureagenesis, and Lipolysis Quantified using the Perfused Mouse Liver Model
In This Article
Summary
Here, we present a robust method for in situ perfusion of the mouse liver to study the acute and direct regulation of liver metabolism without disturbing the hepatic architecture but in the absence of extra-hepatic factors.
Abstract
The liver has numerous functions, including nutrient metabolism. In contrast to other in vitro and in vivo models of liver research, the isolated perfused liver allows the study of liver biology and metabolism in the whole liver with an intact hepatic architecture, separated from the influence of extra-hepatic factors. Liver perfusions were originally developed for rats, but the method has been adapted to mice as well. Here we describe a protocol for in situ perfusion of the mouse liver. The liver is perfused antegradely through the portal vein with oxygenated Krebs-Henseleit bicarbonate buffer, and the output is collected from the suprahepatic inferior vena cava with clamping of the infrahepatic inferior vena cava to close the circuit. Using this method, the direct hepatic effects of a test compound can be evaluated with a detailed time resolution. Liver function and viability are stable for at least 3 h, allowing the inclusion of internal controls in the same experiment. The experimental possibilities using this model are numerous and may infer insight into liver physiology and liver diseases.
Introduction
The liver is an essential organ in metabolism. It plays a key role in the control of whole-body energy balance by regulating glucose, lipid, and amino acid metabolism. The increase in liver diseases worldwide is emerging as a major global health burden, and more knowledge is needed about the pathophysiology and its consequences for liver functions.
Various in vitro models have been developed for research on the liver to complement in vivo studies. Isolated and cultured primary hepatocytes from rodents and humans are widely used. Non-parenchymal cells can be separated from hepatocytes using differential and gradient centrifugation, and the co-culture of different cell types is useful for studying intercellular crosstalk1. Although primary human hepatocytes are considered the golden standard for testing drug toxicity, several studies have shown that the hepatocytes rapidly dedifferentiate in tissue culture resulting in loss of hepatic functions2,3,4. Hepatocyte culture in a 3D spheroid system ameliorates the dedifferentiation, is more stable, and appears to mimic the liver in vivo to a higher degree than the traditional 2D culture systems5. Precision-cut liver slices are another well-established in vitro model that keeps the tissue architecture intact and contains the non-parenchymal cells present in the liver6. More advanced in vitro models include liver-on-a-chip7 and liver organoids8. However, with all these approaches, there is a loss of structural integrity and flow dynamics, including vectorial portal-hepatic vein flow, which likely impacts the generalizability.
The isolated perfused rat liver was first described by Claude Bernard in 18559, and is still used in various scientific fields for studies of liver biology, toxicology, and pathophysiology. Advantages of the perfused liver compared to the above-mentioned in vitro models include the maintenance of the hepatic architecture, the vascular flow, the hepatocyte polarity and zonation, and the interactions between hepatocytes and non-parenchymal cells. Compared to in vivo studies, the perfused liver allows the study of liver metabolism in an isolated manner avoiding extra-hepatic factors carried by the blood and with complete control over the experimental conditions. Several modifications have been made to improve the rat liver perfusion model over the years10,11,12,13. Although mice have been used for isolated perfused liver studies, less literature is available. Here, we present a method for in situ perfusion of the mouse liver by cannulation of the portal vein and the suprahepatic vena cava inferior to study the acute and direct metabolic responses to metabolic substrates and hormones as measured in the hepatic venous effluent from the mouse liver in real-time.
Access restricted. Please log in or start a trial to view this content.
Protocol
All animal experiments were conducted with permission from the Danish Animal Experiments Inspectorate, Ministry of Environment and Food of Denmark (permit 2018-15-0201-01397), and the local ethics committee in accordance with the EU directive 2010/63/EU, the National Institutes of Health (publication No. 85-3) and following the guidelines of Danish legislation governing animal experimentation (1987). This is a terminal procedure, and the cause of death is exsanguination and perforation of the diaphragm under deep anesthesia.
1. Experimental animals
- Obtain mice of desired strain, age, and gender. This study used male C57BL/6JRj mice aged 11-16 weeks. House up to 5 male or 8 female mice per cage with ad libitum access to chow and water and maintain a 12 h/12 h light-dark cycle with lights on from 6 AM to 6 PM.
2. Preoperative preparations
- Make liver perfusion buffer.
- Krebs-Henseleit Buffer. Mix and dissolve 118 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgSO4, and 1.2 mmol/L KH2PO4 in dH2O. Dissolve 1.25 mmol/L CaCl2 in a separate beaker and add the buffer.
- Dissolve 25 mmol/L NaHCO3 and add slowly to the buffer while stirring. Store the buffer at 4 deg. The buffer is stable for at least 1 month.
NOTE: Precipitation may occur if CaCl2 and NaHCO3 are not dissolved individually before being added.
- Filter the perfusion buffer through a 2 µm filter and adjust pH to 7.5 using HCl.
NOTE: This step should be performed on the experimental day, as pH increases over time, even when stored in closed flasks. - Prepare test compounds in a higher concentration than the desired final concentration (e.g., 20x concentration when infused at a rate of 0.175 mL/min via a sidearm pump) in filtered and pH-adjusted Krebs-Henseleit perfusion buffer. If relevant, dilute test compounds in Krebs-Henseleit perfusion buffer with 1% BSA added as a carrier (to avoid adhesion to tubing and glassware, essential for all peptides), filtered through an appropriate size filter.
3. Operation and perfusion
NOTE: An illustration of the perfusion setup used in this study is provided in Figure 1.
- Gas the perfusion buffer (95% O2, 5% CO2) for at least 30 min to supply sufficient oxygen to the liver and to maintain the correct pH from the start of the operation (the bicarbonate buffer system will reach a pH of 7.4 under continuous gassing, see Supplementary Figure 1).
- Anesthetize a mouse by administering ketamine (90 mg/kg) and xylazine (10 mg/kg) by intraperitoneal injection.
- Place the mouse supine on a heated operation table and confirm the lack of reflexes in response to the toe pinch. Spray with 70% ethanol to keep hair from sticking to the scissors. Fixing the mouse to the warm surface helps to increase stability for the following procedures.
- Make an incision with scissors at the base of the abdomen and cut upwards to the ribcage on both sides to expose the abdominal cavity. Move the intestines to the right using a cotton swab, exposing the portal vein.
- Place two ligatures under the portal vein using curved forceps and prepare a loose knot for each ligature.
- Insert a 0.7 mm catheter into the portal vein. When perforated, remove the needle from the catheter and guide the catheter through the vein until the tip of the catheter is close to the liver, as illustrated in Figure 2. Blood flows into the catheter.
- Tighten the ligatures. If the catheter is not filled with blood, fill it up with perfusion buffer to avoid the introduction of an air bubble.
- Attach the perfusion tube and initiate perfusion of the liver with Krebs-Henseleit bicarbonate buffer at 37 °C by starting the roller pump at a perfusion flow rate of 0.8 mL/min. The liver turns pale within seconds.
- Cut the ribcage and diaphragm using scissors. At this point, the animal is euthanized. Place a ligature under the suprahepatic inferior vena cava using fine point forceps. Place a pen, rolled gauze, or another disposable item under the back of the mouse to make the vein more accessible.
- Insert a catheter into the suprahepatic inferior vena cava via the right atrium of the heart. When perforated, remove the needle from the catheter and guide the catheter through the vein until the tip of the catheter is close to the liver. Blood and perfusion buffer runs out immediately.
- Tighten the ligature and attach a tube for the collection of perfusion effluent. Secure all tubes with waterproof tape (sleek tape or similar).
- Use a vessel clamp adaptor to place a vessel clamp across the infrahepatic vena cava immediately above the right renal vein to prevent admixture (Figure 2).
- Increase the perfusion flow rate to 3.5 mL/min and start a timer. Start the pressure recording. Successful perfusion usually results in a pressure of ~10 mm/Hg.
- Cover the liver with a sterile drape moistened with saline and add saline during the experiment to prevent it from drying.
- Collect the perfusion effluent for 1 min and measure the volume. The volume should be approximately 3.5 mL/min. Admixture of blood is not expected at this stage, and the heart does not pump any longer.
- Wait for an equilibration period of 30 min before starting the experiment.
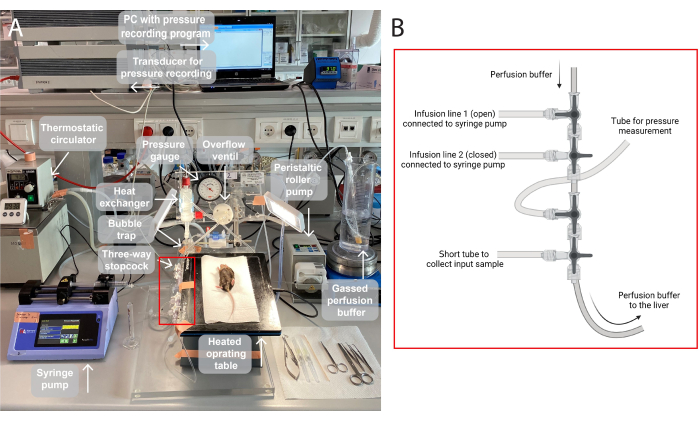
Figure 1: An illustration of the perfusion setup. (A)The operating table is elevated on a tripod stand and heated to 37 °C. The perfusion buffer is gassed (95% O2, 5% CO2), pumped via a peristaltic roller pump, and heated in the heat exchanger with a built-in bubble trap. The system furthermore consists of a pressure gauge and spindle pump for adjustment of the perfusion pressure. The perfusion pressure is continuously recorded and visualized via a transducer on a PC, a pressure recording program. (B) The red box captures the connections of three-way stopcocks. The first three-way stopcock is open for the infusion of a test compound via a syringe pump, and the second is closed. The third is open for continuous pressure measurements. The fourth stopcock may be used to collect input samples, for e.g., gas analysis across the perfused liver. The connectors can be modified as needed for specific experiments requiring more or fewer infusion lines. Please click here to view a larger version of this figure.
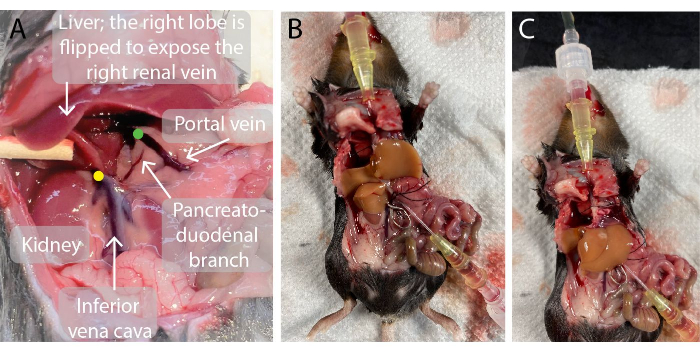
Figure 2: Photos of the mouse abdominal cavity before and during liver perfusion. (A)The green dot indicates the location of the tip of the portal vein catheter. It is important that the tip of the catheter is positioned just below the branching point of the portal vein into the left and right hepatic portal veins but above the pancreato-duodenal branch to avoid leakage. The yellow dot indicates the correct location of the vessel clamp on the infrahepatic inferior vena cava between the right renal vein and the liver to avoid the backflow of blood into the perfused liver. (B, C) A perfused mouse liver with the two catheters inserted in the portal vein (B) and suprahepatic inferior vena cava (C) and the vessel clamp on the infrahepatic inferior vena cava (B). Please click here to view a larger version of this figure.
4. Experiment
- Start the experiment by collecting the first baseline samples using a fraction collector at the end of the equilibration period. Collect samples at the desired time interval and place them on ice immediately.
- Check the bubble trap regularly and refill with perfusion buffer when close to empty.
- Collect a sample of the buffer through a three-way stopcock immediately before it enters the organ and from the collecting catheter inserted in the inferior vena cava (after it has been perfused through the liver).
- Measure the samples with a blood-gas analyzer to confirm that the organ is metabolically active (indicated by an increase in the partial pressure of CO2 and a decrease in pH).
- Repeat steps 4.3-4.4 at the end of the experiment to assess viability throughout the experiment (Supplementary Figure 2).
NOTE: The decrease in oxygen partial pressure does not provide a reliable measure of respiration because of losses of oxygen from tubing, the organ etc. - After 15 min of baseline perfusion, start the first stimulation by infusing a test substance through a three-way stopcock at desired flow rate using a syringe pump (e.g., 20x concentration of the test substance when infused at a rate of 0.175 mL/min via a sidearm pump). Alternatively, switch the baseline buffer to a new (oxygenated and heated) buffer containing the test compound in the final concentration.
- Stop the stimulation and collect baseline samples for 20-30 min before starting the second stimulation.
- At the end of the experiment, infuse an appropriate positive control for 5-10 min.
- After the experiment, excise the perfused liver and weigh it to normalize the output to liver weight. Snap-freeze the liver in liquid nitrogen for potential measurement of protein content to normalize the output to protein content.
5. Biochemical measurements
- Quantify the concentration of the molecule of interest using assays appropriate for measurements in perfusion buffer (in-house or commercially available colorimetric or ELISA assays). The buffer is compatible with most omics-based techniques such as metabolomics and proteomics.
NOTE: In this study, urea was measured based on a colorimetric assay previously described14. Glucose and non-esterified fatty acids were quantified using commercially available kits.
6. Data analysis
- Present the data in XY graphs showing the secretory output over time.
NOTE: It is one of the virtues of the in vitro perfusion system that it is possible to express data as the actual output (concentration × flow rate, e.g., µmol/min) instead of the measured concentration in the output (mmol/L) as in in vivo studies. Consider normalizing the output to the liver weight when comparing different mouse models or to the total protein content (measured by BCA) when comparing control mice to mouse models with obesity or liver diseases where an increase in liver weight may be due to increased fat content. - Present summary data as individual dots per animal representing the mean or total output during baseline and over a stimulation period (typically 15-30 min) or incremental output using the preceding baseline for each stimulation period dependent on the study design.
- Analyze the data using paired t-test (two groups) or one-way ANOVA with repeated stimulations (more than two groups) with an appropriate post-hoc test for multiple testing.
Access restricted. Please log in or start a trial to view this content.
Results
A steady baseline is required to determine whether a stimulus or substrate leads to the release of the molecule of interest. Figure 3A shows an example of a successful experiment. Production of urea in the perfused liver is measured in 2 min intervals and shown as mean ± SEM. The baseline periods preceding each of the two stimulation periods are steady. The mean urea production during the two stimulation periods and the respective preceding baselines are...
Access restricted. Please log in or start a trial to view this content.
Discussion
The isolated perfused mouse liver is a strong research tool for studies of the dynamics and molecular mechanisms of hepatic metabolism. The possibility of minute-to-minute sample collection provides a detailed evaluation of the direct effect of a test compound on the liver. Compared to in vivo studies, the perfused liver allows us to study liver metabolism in an isolated manner avoiding extra-hepatic factors carried by the blood and with complete control over the experimental conditions. The advantages of liver ...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors declare no conflicts of interest relevant to this article.
Acknowledgements
The studies and Nicolai J. Wewer Albrechtsen were supported by Novo Nordisk Foundation Excellence Emerging Investigator Grant - Endocrinology and Metabolism (Application No. NNF19OC0055001), European Foundation for the Study of Diabetes Future Leader Award (NNF21SA0072746) and Independent Research Fund Denmark, Sapere Aude (1052-00003B). Novo Nordisk Foundation Center for Protein Research is supported financially by the Novo Nordisk Foundation (Grant agreement NNF14CC0001). Figure 1B was created with biorender.com. We thank Dr. Rune E. Kuhre (Novo Nordisk A/S) for fruitful discussions on the perfused mouse liver.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 3-way stopcock | BD | 394601 | |
| Altromin breeding diet | Altromin Spezialfutter | 1319 | |
| Calcium chloride dihydrate (CaCl2·2H2O) | Sigma | C8106 | |
| Catheters (0.7 mm) | BD | 381812 | |
| Filter paper (pore size 2.0 µm) | Millipore | AP2029325 | |
| Glucose kit | QuantiChromTM | DIGL-100 | Low concentration protocol |
| Ketamine | MSD Animal Health | 511485 | 90 mg/kg |
| Ligature (black sterile silk) | Agnthos | 14739 | |
| Magnesium sulfate (MgSO4) | Sigma | 230391 | |
| Non-esterified fatty acids kit | Fujifilm Wako Chemicals | NEFA-HR(2) | |
| Operating table, heated on tripod stand, type 873 | Harvard Bioscience, Inc. | 733776 | |
| Potassium chloride (KCl) | Sigma | P9541 | |
| Potassium dihydrogen phosphate(KH2PO4) | Merck | 1.04877 | |
| Roller Pump, with four channels | Harvard Bioscience, Inc. | 730100 | |
| Sleek tape | Mediq danmark | 4001910 | |
| Sodium bicarbonate (NaHCO3) | Sigma | S5761 | |
| Sodium chloride (NaCl) | Sigma | S1679 | |
| Thermostatic Circulator | Harvard Bioscience, Inc. | 730125 | Bath Volume 3 L, 230 V/50 Hz |
| Tubing | Tygon | E3603 | Inner diameter 1.59 mm, outer diameter 3.18 mm |
| Universal perfusion system | Harvard Bioscience, Inc. | 732316 | Basic unit uniper UP-100, type 834 |
| Vamin | Fresenius Kabi | B05ABA01 | Mixed amino acids |
| Vessel clamp adaptor | Deutsche Biomedical | DBC1002 | |
| Vessel clamps | Deutsche Biomedical | DBC1005 | |
| Windkessel | Harvard Bioscience, Inc. | 732068 | |
| Xylazine | Rompun Vet | 530701 | 10 mg/kg |
References
- Bale, S. S., Geerts, S., Jindal, R., Yarmush, M. L. Isolation and co-culture of rat parenchymal and non-parenchymal liver cells to evaluate cellular interactions and response. Scientific Reports. 6, 25329(2016).
- Lauschke, V. M., et al. Massive rearrangements of cellular MicroRNA signatures are key drivers of hepatocyte dedifferentiation. Hepatology. 64 (5), 1743-1756 (2016).
- Seirup, M., et al. Rapid changes in chromatin structure during dedifferentiation of primary hepatocytes in vitro. Genomics. 114 (3), 110330(2022).
- Gupta, R., et al. Comparing in vitro human liver models to in vivo human liver using RNA-Seq. Archive of Toxicology. 95 (2), 573-589 (2021).
- Bell, C. C., et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function, and disease. Scientific Reports. 6, 25187(2016).
- Dewyse, L., Reynaert, H., van Grunsven, L. A. Best practices and progress in precision-cut liver slice cultures. International Journal of Molecular Sciences. 22 (13), 7137(2021).
- Li, X., George, S. M., Vernetti, L., Gough, A. H., Taylor, D. L. A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab on a Chip. 18 (17), 2614-2631 (2018).
- Broutier, L., et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nature Protocols. 11 (9), 1724-1743 (2016).
- Bartošek, I., Guaitani, A., Miller, L. L. Isolated Liver Perfusion and its Applications. , Raven Press. New York. (1973).
- Gores, G. J., Kost, L. J., LaRusso, N. F. The isolated perfused rat liver: conceptual and practical considerations. Hepatology. 6 (3), 511-517 (1986).
- Mischinger, H. J., et al. An improved technique for isolated perfusion of rat livers and an evaluation of perfusates. Journal of Surgical Research. 53 (2), 158-165 (1992).
- Vairetti, M., et al. Correlation between the liver temperature employed during machine perfusion and reperfusion damage: role of Ca2. Liver Transplantation. 14 (4), 494-503 (2008).
- Ferrigno, A., Richelmi, P., Vairetti, M. Troubleshooting and improving the mouse and rat isolated perfused liver preparation. Journal of Pharmacological and Toxicological Methods. 67 (2), 107-114 (2013).
- Zawada, R. J. X., Kwan, P., Olszewski, K. L., Llinas, M., Huang, S. -G. Quantitative determination of urea concentrations in cell culture medium. Biochemistry and Cell Biology. 87 (3), 541-544 (2009).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved