Method Article
Enzymatic Modification and Flow Cytometry Assessment of Yeast Surface Displayed Proteins
* These authors contributed equally
In This Article
Summary
Here, we introduce a protocol for using yeast surface-displayed substrates for enzymatic modification assays. The platform was demonstrated using the analysis of the dephosphorylation activity of tyrosine phosphatase SHP-2 against one of its substrates as a representative enzymatic modification assay.
Abstract
Yeast surface display is a genotype-phenotype linkage strategy that empowers high-throughput screening of protein function. Traditionally, yeast surface display has been applied to the evolution of new binding proteins, with flow cytometry used to assess and sort by levels of binding strength. Recently, there has been growing interest in applying yeast surface display for screening enzymatic modification of substrate variants, with additive (e.g., phosphorylation) or subtractive (e.g., proteolysis) modifications providing a phenotype readable by flow cytometry. Such modifications are regularly applied using intracellular co-localization, but the ability to achieve extracellular enzymatic modification of displayed substrates could open many more reactions to investigation. Here, we describe techniques for designing and applying screening assays for extracellular enzymatic modification to candidate substrates displayed on the yeast surface and subsequent evaluation using flow cytometry analysis. We provide these protocols in the context of phosphatases dephosphorylating yeast displayed substrates containing phosphorylated tyrosine residues and comment on how this applied framework can be adapted to developing screening assays for other enzyme-substrate pairs.
Introduction
The understanding of the interactions between enzymes and their targets has become an increasingly interesting area of research due to its necessity in the biological characterization of the pathways controlling cellular homeostasis and disease development1,2. Enzymes are responsible for the catalysis of many of the reactions that maintain biological life, controlling necessary pathways such as cellular metabolism3,4, signaling5, and even fundamental processes like genome repair6,7. Due to their role in these processes, their interactions also play a role in the development of many diseases, as deviations in their activity can cause severe dysregulation in cell activity, causing apoptosis or proliferation of harmful cancer cells2. The study of enzymatic activity has had important applications in the development of new therapeutics8,9, requiring assays that are tailored to each specific enzyme-substrate interaction10. Multiple enzymatic assays have been established as standard protocols for the evaluation and characterization of these interactions. Assays developed to analyze enzymatic interactions are classified into detection assays that monitor binding for activation/inhibition11 or assays that monitor substrate modification by enzymes12.
One major role of enzymes is regulating cell behavior. Signal transduction, the intracellular response of a cell to an extracellular trigger13, is responsible for cell survival and functionality. Cell proliferation, differentiation, and many other functional processes all involve signaling pathways with enzymatic interactions governing them14,15. Enzymes catalyze post-translational modifications, which often modulate the massive signaling networks responsible for the correct transmission of extracellular messages16. Protein phosphorylation is the most common post-translational modification, ubiquitous in cell signaling and multiple other cellular pathways. Consequently, protein kinases have emerged as a significant proportion of potential therapeutic targets due to their critical regulatory role17. Phosphatases are the natural modulatory molecules for phosphate-based cell signaling complexes18,19, having the capacity to remove phosphate residues from their target proteins20. In the last decade, phosphatases have become a major therapeutic target for cancer treatment21 and inflammatory diseases22 based on their involvement in the regulation of downstream signaling pathways in multiple cell types. Together, protein kinases and phosphatases provide a breadth of interactions, which can be studied through the development of specific enzymatic assay protocols.
Yeast surface display has been used as a tool for the characterization and evaluation of enzymatic activity23,24. It provides a high-throughput platform for the screening of post-translational modification processes when combined with endoplasmic reticulum sequestration strategies25,26. This allows kinase-substrate pairs to be co-localized and retained in the endoplasmic reticulum through binding to KDEL receptors27, where phosphorylation of the substrate can occur at increased rates due to the proximity between kinases and their targets. The KDEL receptor binding is mediated by a C-terminal FEHDEL endoplasmic reticulum retention sequence shown to have a stronger retention ability than other HDEL sequences25,28. The phosphorylated substrate is then anchored to the yeast surface for its subsequent evaluation through flow cytometry29. Currently, there are no generalizable protocols established for the enzymatic modification of substrates displayed on the yeast surface. We expand on the capacities of yeast surface display by taking advantage of the extracellularly expressed phosphorylated substrate variants and modifying them through dephosphorylation by their known phosphatase. Flow cytometry analysis then provides a platform for the phenotypic evaluation of the aforementioned substrates through the measurement of alterations in phosphorylation median as a consequence of the incubation with the known phosphatase. This provides an adaptable method for post-translational modification of surface-displayed proteins while also providing a method for enzymatic modification analysis of interactions when using the yeast surface display platform.
We present techniques for the development and application of an enzymatic modification assay that describes the introduction of a kinase-substrate interaction into the yeast surface display platform, the co-incubation of the expressed phosphorylated substrate with a recombinant phosphatase, and the subsequent analysis of the dephosphorylation activity through flow cytometry. In this report, this is accomplished by co-localizing the cytoplasmic domain of CD28 with lymphocyte kinase (LCK) in the yeast endoplasmic reticulum, followed by display of the phosphorylated CD28 on the yeast surface and subsequent dephosphorylation by Src homology region 2 domain-containing phosphatase-2 (SHP-2). A pan anti-phosphotyrosine antibody (in this study, 4G10), which detects phosphorylated tyrosine residues in a wide variety of peptide sequences, is used for quantification of phosphorylation level as a function of phosphatase treatment. The detailed process provides a generalizable approach for investigating enzyme-substrate interactions; a prospective way of studying enzymes and substrates in purified fashion.
Protocol
1. Cell growth of yeast harboring plasmid and induction of protein expression
- Following the recipe described in Table 1, prepare the media required for growth of non-plasmid containing yeast (YPD), plasmid-containing yeast cell growth (SD-CAA) and the induction of protein expression (SRG-CAA) as well as SD-CAA plates.
- Transform yeast display plasmid DNA containing the kinase-substrate pair into EBY-100 yeast cells through the lithium cation-based method30,31, commonly adopted for use in yeast transformation kits from a variety of manufacturers.
NOTE: Electroporation32 or other preferred yeast plasmid transformation techniques can be used depending on plasmid construct being transformed. - Prepare a 14 mL culture tube with 10 mL of YPD media. Inoculate EBY-100 cells and grow in a shaking incubator at 30 °C, 300 rpm until the culture reaches an optical density (OD600nm) of 0.8-1.0 (8 x 106- 1 x 107 cells/mL).
NOTE: OD600nm is measured by preparing 3 mL sample cuvettes containing 1:10 dilutions of yeast cultures in their respective media and 3 mL blank cuvettes containing the media used for sample dilutions. The OD600nm program on the spectrophotometer is used to first measure the blank cuvette, then each sample cuvette by setting the corresponding dilution prepared for each sample. 1 OD600nm corresponds to 1 × 107 yeast/mL. - Harvest the cells by centrifuging the culture at 1,000 x g for 3 min, and wash with the 1st washing solution provided in the yeast transformation kit, or TE (10 mM Tris-HCl and 1.0 mM EDTA)31.
- Pellet the cells again at 1,000 x g for 3 min and resuspend in 1 mL of the transformation buffer provided in the yeast transformation kit, or sterile water. The cells should be aliquoted into 50 µL aliquots and can be stored at -80 °C for up to 6 months.
- For plasmid transformation, one aliquot per plasmid is prepared and thawed on ice, then, 0.5-1.5 µg of plasmid DNA containing the yeast display construct is added directly to the cells. 0.5 mL of the transformation solution provided in the yeast transformation kit is added, or 0.5 mL of a sterile 50% polyethylene glycol and 0.1 M LiOAc solution31. Combine the mixture of cells, plasmid DNA, and transformation solution thoroughly by pipetting.
- Incubate transformation mixture statically for 30-60 min at 30 °C, vortex mixture at 15 min intervals. Harvest cells by centrifuging at 1,000 × g for 3 min.
- Prepare a 14 mL culture tube with 4.5 mL of SD-CAA media. Resuspend the cells containing the desired plasmid in 500 µL of SD-CAA and inoculate the prepared 4.5 mL.
- Being careful not to pierce the agar, distribute 50 µL of the 5 mL of inoculated culture onto a SD-CAA plate and incubate statically at 30 °C for 48 h to determine the transformation efficiency.
- Incubate the 5 mL of SD-CAA cell culture in a shaking incubator at 30 °C, 300 rpm for at least 18 h. Monitor optical density (OD600nm) after 16 h and 20 h. Once the sample has grown to a sufficient optical density not exceeding 6, centrifuge the culture for 3 min at 2,500 × g. Discard the supernatant without disturbing the yeast pellet.
- Resuspend the yeast pellet in SRG-CAA to a final OD600nm less than 1 (<1 × 107 yeast/mL).
- Incubate the yeast culture in a shaking incubator at 30 °C, 300 rpm for at least 8 h but no longer than 24 h.
NOTE: Induction of protein expression in yeast cells can be varied anywhere from 20-37 °C. 30 °C is suitable for synthesis of kinase/substrate pairs29,33 but can be adjusted if deemed necessary for the specific proteins being studied. - Measure OD600nm to determine the cell density.
NOTE: The protocol can be stopped at this point by storing the yeast cultures at 4 °C.
2. Biotinylation of 4G10 anti-phosphotyrosine antibody
- Resuspend a 2 mg vial of PEG4-NHS-Biotin to a final concentration of 5 mM by adding 680 µL of sterile PBS.
NOTE: Resuspension of PEG4-NHS-Biotin should be done freshly immediately before the biotinylation reaction is carried out. NHS hydrolyzes in aqueous solution. Use of sterile PBS and vials are important for the preparation of reagents for long-term storage and for use in cell-based assays to mitigate any potential effects of contaminants on reagent viability or the sensitive assays being performed. - Based on the 4G10 antibody concentration, add 100 µg of antibody into a sterile 1.7 mL vial.
- Add 1 µL of the prepared 5 mM PEG4-NHS-Biotin from step 2.1 to the vial holding the 100 µg of 4G10 antibody to achieve a molar ratio of biotin to antibody of 7.5:1. Pipette the mixture gently to homogenize the reaction.
- Incubate the reaction at room temperature with constant rotation for at least 2 h.
- Follow the manufacturer's protocol for 0.5 mL spin desalting columns to exchange the buffer from the biotinylated 4G10 (B-4G10) into PBS.
NOTE: Desalting columns with a molecular weight cutoff (MWCO) of 7 kDa are commonly used for biotinylation of antibodies to allow unreacted biotin and other small molecules to be removed while retaining the larger antibody. - Dilute the B-4G10 antibody to a final concentration of 1 µM in PBSA (PBS with 1 g/L bovine serum albumin). Aliquot the B-4G10 antibody into smaller volumes to prevent repeated freeze/thaw cycles.
NOTE: Biotinylated antibodies can be stored at 4 °C for daily usage for up to 3 months without losing significant efficiency. Store the rest of the aliquots not in use at -20 °C for a maximum of 2 years.
3. Dephosphorylation of substrates expressed on the yeast cell surface
- Prepare the 2x working buffer solution as previously described in the literature34.
NOTE: 2x is recommended to facilitate the measurement of ingredients needed. - Prepare the working buffer for the samples in a 1.7 mL vial by diluting the 2x buffer solution prepared in step 3.1 1:2 in deionized water.
NOTE: The total reaction volume for each sample will be 20 µL, and each sample will require anywhere between 10 µL and 18 µL of working buffer. Prepare enough working buffer for all samples or controls. - Following the recommended sample preparation described in Table 2, label 1.7 mL vials with the corresponding control or sample name.
- Based on the OD600nm measured in step 1.9, calculate the volume of culture necessary to recover two million (2 × 106) yeast cells from the corresponding yeast culture for each sample.
- Add the volume of yeast culture calculated in the previous step into the 1.7 mL vial for each sample.
- Centrifuge the vial for 1 min at 4,500 × g. Carefully remove supernatant using a micropipette and discard as biohazardous waste.
- Resuspend the pelleted cells in 1 mL of PBSA and repeat step 3.6.
NOTE: It is important to remove as much supernatant as possible without disturbing the pelleted cells. - Based on its stock concentration, calculate the volume of recombinant human SHP-2 required to have a final concentration of 1,000 nM in a 20 µL total reaction volume.
NOTE: Recombinant SHP-2 should be aliquoted in small volumes so that any protein used in each assay has not gone through more than two freeze-thaw cycles. Any SHP-2 leftover from an aliquot after all samples have been prepared for an assay should be discarded.
Stock concentrations of recombinant enzymes can vary depending on lot number. Recombinant SHP-2 is commonly formulated at a stock concentration of 0.2-0.4 mg/mL. For a stock concentration of 0.324 mg/mL SHP-2, this corresponds to a stock concentration of 4.696 µM (SHP-2 has a molecular weight of 69 kDa). 4.26 µL of the SHP-2 stock in a 20 µL reaction results in a final reaction concentration of 1,000 nM SHP-2. - Add 7.7 mg of DTT into 10 mL of deionized water prepared in a 15 mL conical to create a 5 mM DTT solution. If equipment limitations make it difficult to weigh milligrams, add 0.77 g of DTT to 10 mL of deionized water, then perform a 100x dilution to create the 5 mM DTT solution used for the assay.
NOTE: The DTT solution can be prepared in higher concentration stock solutions to be diluted to 5 mM if it is not possible to measure milligram-scale quantities using the equipment available. DTT solution needs to be prepared fresh preceding cell resuspension in working buffer due to its tendency for hydrolyzing, making it unstable over long periods when diluted in water. - Resuspend the pelleted cells in the working buffer prepared in step 3.2 so that the final reaction volume in each sample or control is 20 µL.
NOTE: The amount of working buffer added should be calculated based on the amount of DTT (2 µL) and SHP-2 (calculated in step 3.8) that will be in each sample. - Add 2 µL of the 5 mM DTT solution prepared in step 3.9 to each sample or control for a final reaction concentration of 0.5 mM DTT.
- Add the volume of SHP-2 calculated in step 3.8 to each sample for a final volume of 20 µL and gently mix using a micropipette.
- Wrap the sample vial lids in parafilm to prevent leakage or cross-contamination.
- Incubate the samples at 37 °C for 2 h on a rotor at a constant speed.
- Remove samples from the rotor and stop the reaction by adding 1 mL of PBSA to each sample.
- Repeat step 3.6.
4. Cell labeling and flow cytometry analysis of dephosphorylated substrates
- Resuspend samples from step 3.16 in a 20 µL mix of their corresponding primary reagents as described in Table 2. Incubate samples for 20 min at room temperature.
NOTE: All reagent concentrations used have been calculated to be in excess with regard to the number of proteins expressed on the surface of yeast cells. The calculation assumes the expression of 10,000 proteins/cell from all 2 x 106 yeast in a sample35, when routinely only ~50% of them do. Table 3 shows the excess labeling reagent ratio for each of the reagents expressed in Table 2, calculated as follows:
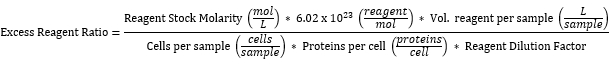
- Centrifuge samples at 4,500 × g for 1 min and discard the supernatant as biohazardous waste.
- Wash cells once by resuspending in 1 mL of PBSA. Repeat step 4.2.
- Resuspend samples in a 20 µL mix of their corresponding secondary reagents as described in Table 2. Incubate samples for 15 min in the absence of light.
- Repeat step 4.2.
- Repeat step 4.3.
- Resuspend the washed samples in 300-500 µL of PBSA and transfer into 5 mL polystyrene tubes to analyze immediately using an appropriate flow cytometer.
NOTE: If samples need to be transported, keep on wet ice. It is not recommended, but samples can be stored at 4 °C for a maximum of 2 h as wet pellets. - After performing necessary startup and preparation of the cytometer for a new experiment, click on the New Experiment button within the File menu, name the experiment, and click Save to ensure data acquired is saved in the desired file path.
- Select the dot-plot icon within the upper toolbar to create two or more dot-plots for each sample to be run. For one of the dot-plots, select the X-axis name to display the FSC-A channel and the Y-axis name to display the SSC-A channel. This plot shows the Side Scatter - Area versus Forward Scatter - Area and is used to gate yeast cells for further analysis.
- On another dot-plot, select the X-axis name to display the channel in which the secondary reagent targeting the primary anti-epitope tag antibody fluoresces. Select the Y-axis name to display the channel in which the streptavidin secondary reagent fluoresces. This plot will show only the events gated from the side versus forward scatter plot as yeast cells and is used to display tyrosine phosphorylation on the Y-axis and substrate surface expression on the X-axis.
NOTE: The secondary reagent targeting the primary anti-epitope tag antibody fluoresces in the FITC (AF-488) channel and the streptavidin secondary reagent fluoresces in the AF-647 channel in this example. Channels used may vary depending on the primary and secondary reagents used during labeling. - Place each sample tube in the tube holder of the cytometer and select Run for the cytometer to begin loading the sample and acquiring data. Adjust events to display, events to record, time to record, and sample flow rate as necessary.
- Define a gate surrounding the healthy yeast cells in the SSC-A vs FSC-A plot created in step 4.9. Figure 1 illustrates a descriptive representation of the gating strategy to apply.
NOTE: On the SSC-A versus FSC-A plot, 100,000 events to display, and 50,000 events to record within the defined yeast gate is a good guideline to visualize and collect sufficient data for further analysis. More stringent gating strategies to select for singlet yeast can be applied based on a plot of Forward Scatter-Height versus Forward Scatter-Area as recently reported36. The gating strategy shown in Figure 1 corresponds to a less stringent approach, including singlet and some doublet yeast through scatter gating. - Record fluorescence of all control samples using a flow cytometry analyzer. Control samples are routinely collected first to help define a gating strategy, as described below in step 4.14.
- Define a gating strategy for your plot created in step 4.10 prior to analyzing treated samples. Figure 1 illustrates a descriptive representation of the gating strategy to apply.
- Record fluorescence of dephosphorylated samples using the flow cytometer and the gating strategy defined in steps 4.12 and 4.14.
- Analyze flow cytometry data acquired using a flow cytometry analysis software.
- Evaluate dephosphorylation by measuring and comparing the Y-axis median of cells expressing protein on their surface and the baseline phosphorylation provided by non-displaying cells between samples and controls. Calculate the percent median phosphorylation difference as follows:

Results
Flow cytometry analysis from an individual replicate of our model system incubated for 2 h without (Figure 1A) and with (Figure 1B) 1,000 nM SHP-2 reveals a median phosphorylation difference of 63.6%, which is defined as the ratio of Y-axis median (phosphorylation) from all surface displayed events minus the baseline phosphorylation signal defined as the Y-axis median of the non-displayed events between the treated sample and the non-treated control as described in the equation defined in protocol step 4.12. Prior to the analysis of the medians, samples were gated based on their size (Forward Scatter) and complexity (Side Scatter) to encompass a healthy group of cells. Under the defined conditions, the sample dephosphorylation should be distinguishable in plain sight (Figure 1C).
The assay is defined by a straightforward procedure consisting of four main methods (Figure 2A). The yeast surface display system that we use and have published previously is based on a plasmid containing a bidirectional promoter with the capacity for simultaneous inducible expression of a kinase-substrate pair consisting of the cytoplasmic tail of CD28 and the tyrosine kinase LCK (Figure 2B)29. Co-localization of the translated proteins is directed by an endoplasmic reticulum targeting signal peptide and the post-translational modification forced by increased residence time resulting from a C-terminal ER retention sequence. Secretion of the phosphorylated substrate fused to Aga2p leads to surface expression (Figure 2C). The substrate is designed to be flanked by two epitope tags, which allows the extracellular confirmation of successful translation and subsequent surface expression. Incubation of the substrate displaying yeast cells with the phosphatase of interest (in this case, tyrosine phosphatase SHP-2) allows the analysis of enzyme modification through the decrease of substrate-bound phosphate (Figure 2D).
Even though optimal conditions have been defined for the model system presented, the generalizability of the assay permits diversification of the proteins to be analyzed. The optimal conditions for the assay were defined through a series of titrations where different combinations of time and concentration of phosphatase were evaluated in quadruplicate (Figure 3). Data analyzed demonstrated statistical significance through a two-way ANOVA with replication (p < 0.05). The chosen conditions of 2 h and 1,000 nM (48.8% ± 10%) offered an approximate 50% median phosphorylation difference while maintaining statistical significance when compared to its 750 nM counterpart at 2 h (p < 0.05) based on a t-test analysis with unequal variance. The t-test also revealed no significant difference from the 2 h and 1,000 nM result by increasing the time by 1 h under any of the concentrations that provided an approximate median phosphorylation difference percent (p > 0.05 for 500 nM, 750 nM, and 1,000 nM at 3 h).
Tukey HSD post hoc analysis reveals that all mean comparisons between incubation periods across all concentrations are significantly different apart from 1 h to 2 h. When comparing the multiple concentrations tested, we only observe statistically significant mean differences when comparing 250 nM to all other concentrations, indicating that comparable levels of phosphatase activity are expected within groups, except for 250 nM. Despite observing a 20% difference when the samples were treated at 4 h and 1,000 nM SHP-2 (22.1% ± 5.5%), in comparison to the optimal conditions (t-test, p < 0.05), we decided to not pursue this combination due to reduced surface expression and diminished yeast health from the long incubation with DTT. We hypothesize this is caused by the reducing conditions of the working buffer, which is needed for proper SHP-2 phosphatase function.

Figure 1: Flow cytometry analysis of the model system. Density plots displaying Forward Scatter (X-axis) versus Side Scatter gating (Y-axis) (left) and dot plots displaying surface expression through substrate C-terminal epitope tag labeling (X-axis) versus substrate phosphorylation (Y-axis) (center) of CD28 cytoplasmic domain incubated for 2 h (A) without SHP-2 and (B) with the optimal concentration of SHP-2 defined as 1,000 nM. Y-Median was measured within the defined gates encompassing surface expressed cells (green) only as a relative phosphorylation measurement. Non-displaying signal events were gated to define the background Y-Median measurement (grey). (C) Dot plot overlay of samples showing plain sight difference in Y-median. Please click here to view a larger version of this figure.

Figure 2: Enzymatic modification of yeast surface displayed proteins. (A) Assay schematic showcasing the critical steps within the four methods described: preparation of samples with phosphatase (tan) for enzymatic assay, incubation for desired enzymatic activity, cell washing and labeling for detection of activity, and flow cytometry analysis and data collection. (B) Gene schematic representing a general structure of the cassette used for endoplasmic reticulum sequestration of an enzyme-substrate pair and surface expression of the substrate. (C) Graphical representation of endoplasmic reticulum co-localization of a kinase-substrate pair (Left) followed by secretion of the post-translationally modified substrate displayed on the yeast surface and labeled with an anti-phosphotyrosine antibody (blue) and an anti-epitope tag antibody (pink) to confirm surface expression (Right). (D) Incubation of yeast cells with phosphatase (tan) removes the phosphate group from the displayed substrate, disrupting anti-phosphotyrosine antibody labeling, facilitating enzymatic modification analysis through flow cytometry. Please click here to view a larger version of this figure.

Figure 3: Phosphatase activity and time titration. Yeast cells were exposed to multiple combinations of time and phosphatase concentration followed by flow cytometry analysis. All treated samples were compared to a control incubated for the same length of time and buffer conditions without SHP-2. Percent median phosphorylation difference was defined as the ratio of Y-median from surface displayed events minus the baseline noise signal provided by the Y-median in non-displaying events in SHP-2 containing samples, divided by the same relation in their respective control. Null hypothesis is rejected when significant differences are observed on comparing changes between time and concentration groups using two-way ANOVA (p < 0.05). The effect of the variables described over the percent median phosphorylation difference is independent from each other (Interaction p > 0.05). Tukey's HSD test was performed for post hoc analysis for further information on the significance of difference across overall incubation time and concentration groups, and a series of t-tests assuming unequal variance were used to define statistical significance of individual groups at a specified time and concentration. Data are presented as mean ± standard deviation of four replicates. Please click here to view a larger version of this figure.
Table 1: Yeast growth and protein induction media preparation guidelines. Tabular description of the mass required from each chemical component to formulate 1 L of Selective Yeast Growth Media, Selective Yeast Protein Induction Media and Yeast Extract Peptone Dextrose Media. Once the medium described has been mixed properly, filter-sterilize prior to its use. Additional instructions are included for making Selective Yeast Growth Media Plates. Please click here to download this Table.
Table 2: Recommended sample preparation for substrate dephosphorylation and antibody labeling strategy. Tabular description of the samples required for the measurement of enzymatic modifications of phosphorylated substrates displayed on the surface of yeast. The sample preparation and following antibody labeling strategy is specified for both the required controls and each sample to be analyzed, including corresponding dilutions of labeling reagents. Please click here to download this Table.
Table 3: Antibody to protein excess ratio calculations. Tabular description of the theoretical number of antibodies available per protein expressed on the surface of a yeast cell while being labeled for flow cytometry. The expressed theoretical number assumes that 100% of the yeast cells express 10,000 proteins on their surface to ensure antibody excess and the excess ratio calculations are based on the reagent stock concentration displayed in the table and acquired from the provider. Please click here to download this Table.
Supplementary Table S1: Amino acid sequence of construct cassettes. Tabular representation of the amino acid sequence for the construct cassettes located on both sides of the Gal 1-10 promoter. Highlighted sequences correspond to their color-coded descriptions. The Gal-10 side of the plasmid is represented as a reverse amino acid sequence translation to facilitate its understanding. All characters left in black correspond to the amino acid translation of restriction enzyme digestion sites used to provide modularity to the construct. Please click here to download this File.
Discussion
The protocol presented allows for the analysis of enzymatic interactions using the extracellular display of proteins on the yeast surface. Incorporating endoplasmic reticulum sequestration into the surface-display plasmid used introduces the capacity to analyze specific interactions between enzymes and post-translationally modified substrates extracellularly due to the intracellular interactions that can be designed to occur27,29. The previously established enzymatic interaction assays using yeast surface display involve the expression of the proteins of interest intracellularly, with surface display solely being a tool for detection of intracellular interactions that occur between the target proteins23,25,29.
This protocol builds onto that platform by moving the targeted enzymatic interactions to the extracellular environment, which introduces additional flexibility in both the enzymes that can be studied, and the environment that their activity is monitored in. The studied interactions occurring extracellularly gives researchers the opportunity to tailor the incubation environment to be more optimal for enzymatic activity, expanding the enzymes that could be studied whose activities are hindered in the yeast endoplasmic reticulum, which is a heavily oxidizing environment37. Furthermore, the ability to titrate the concentration of enzymes with respect to a given substrate allows for specific enzymatic activity assays that could not be performed intracellularly due to hypothesized reaction rate saturation during sequestration.
Within the protocol, there are several critical steps to be noted to ensure that the desired outcome will be observed. Successful transformation of the studied constructs into yeast is essential for optimal results from the following steps. Accurate monitoring of optical density should be done to track the healthy growth of cultures and ensure they do not overgrow prior to protein induction or sample preparation. The log phase of yeast cell growth encompasses the period where protein production is the highest, whereas in the stationary phase, the mechanisms responsible for protein production become arrested38. Keeping this in mind, optical density measurements provide an accurate measurement of the growth phases the yeast cultures are in, and steps such as protein induction and preparation for assays should be done outside of the stationary phase or when cultures become overgrown (OD600nm < 6).
For the enzymatic modification assay, the incubation environment described was specific to the studied enzyme, SHP-2 and the enzymatic activity that was being performed, dephosphorylation. DTT was used for the reducing environment it provides during incubation with SHP-234. Therefore, it is important to measure accurate concentrations of the chemicals used to modify the incubation environment in the enzymatic assays to ensure consistent enzymatic activity between samples and experiments. SHP-2 was used as a recombinant protein, and it is critical to regulate temperature during the different steps of handling the enzyme. For a successful assay, the enzyme should not have gone through more than two freeze-thaw cycles and should be on ice during the preparation of each sample. It is then imperative to aliquot the recombinant enzyme into a sufficient volume to satisfy the assay requirements. During the actual incubation, temperature needs to be strictly controlled at the optimal temperature for the enzyme, 37 °C in this case, with constant movement from a rotor to ensure homogeneity of the incubation mixture.
The overall method for the analysis with recombinant enzymes required modifications specific to the interaction between SHP-2 and the surface-displayed phosphorylated substrate. Adapting the protocol to other extracellular enzyme-substrate interactions involves modification of sequences used, the activity buffer environment, and the reagent used for detection. For assaying other kinase-substrate-phosphatase interactions, adaptation involves replacing the protein sequences for a kinase-substrate pair into their respective positions in the construct cassette described in Supplementary Table S1. The protein sequence of the substrate together with at least the kinase domain of the kinase of interest should be included in the plasmid, and the phosphatase targeting the produced phosphorylated substrate should be in the form of a recombinant protein. The representative interaction between LCK, CD28, and SHP-2 provides an example of using the designed endoplasmic reticulum sequestration in the construct cassette as a tool for producing post-translationally modified proteins to be investigated extracellularly with their targeting enzyme. Substrates of interest that do not need to go through post-translational modifications (e.g., substrates that could be phosphorylated extracellularly using added kinase) can be expressed on the yeast surface without a paired enzyme within the construct cassette. In this case, the protein sequence for the kinase described in Supplementary Table S1 would be removed with just the sequence of the substrate being included in the construct cassette. We do note from our prior experience that co-localization of a serine-threonine kinase with a known substrate resulted in display of substrate that was not phosphorylated (Ezagui and Stern, unpublished data), so rigorous testing of successful enzymatic modification must be conducted prior to applying extracellular phosphatase. We have previously published a protocol for kinase-substrate co-localization that may be helpful for this qualifying step39.
Kinases and phosphatases often contain unpaired cysteine residues which, when oxidized, form disulfide bonds within the protein or across proteins, which can disrupt the catalytic activity of the protein due to conformational change40,41. Understanding of this protein biochemistry is essential for determining the proper reaction environment for enzymatic modification. As a result, a reducing agent needs to be supplemented to the incubation environment to ensure the recombinant protein used remains active. DTT is a common reducing agent used for these purposes, but the concentration in the assay must be optimized. Using too high of a DTT concentration hinders the display of the substrate on the yeast surface, as the Aga1p and Aga2p anchors are held to each other through disulfide bonds, which are reduced in the presence of DTT42. The concentration of DTT was adjusted to the minimal concentration that would allow for a relative maximum phosphatase activity without having detrimental effects on the surface display of substrates42. The incubation environment for whichever enzyme is being assayed should be optimized to ensure the retention of enzymatic activity when used in this assay. If an enzyme requires a reducing environment considerably stronger than the 0.5 mM DTT used in this assay, the platform is limited by the reduction in surface display and may not be optimal for the specific enzymatic assay desired. Similarly, the 2x buffer used during the incubation step in this protocol was included from prior research into acceptable buffers promoting SHP-2 activity, and similar research should be performed for formulating an incubation buffer for any other enzyme being used for this assay34. Starting points for crafting these buffers can include literature search for successful in vitro applications of the enzyme of interest or the enzyme manufacturer's recommendation for an activity buffer. The recombinant enzyme being used should be titrated specifically for this assay to identify an acceptable concentration and incubation time, which allows for the targeted enzymatic activity to occur before data collection.
For adaptation of this protocol to other types of enzyme-substrate interaction, new fluorescent detection reagents will need to be selected and titrated for sensitivity. Other studies have demonstrated examples of this, including the use of epitope tag-targeted antibodies to detect the presence or absence of peptide substrates after protease treatment23,25 and acetylation-sensitive antibodies to detect modifications to histone proteins43. For qualifying these reagents, a positive control (one that verifiably demonstrates the modification of interest) and a negative control (one that verifiably demonstrates a lack of the modification of interest) must be established. This could be done through yeast surface display of a construct that has previously demonstrated the modification of interest, or in some cases could be established through immobilization of recombinant proteins or peptides. For example, in the case of phosphorylation (and many other post-translational modifications of interest), peptides of known sequence could be synthesized with either phosphotyrosine (positive control) or unmodified tyrosine (negative control) and a C-terminal biotin that would enable immobilization of the peptides on streptavidin-coated beads. The peptide-coated beads could be labeled with the modification-specific antibody and assessed for specificity and sensitivity of detection using flow cytometry with methods similar to those described in Section 4. Different dilutions of antibody should be used to find a concentration that provides maximum signal of the positive control, minimal signal of the negative control, and balances sufficient fold-excess of antibody per modified protein (using the equation in the note from Step 4.1) with economic considerations for number of experiments to be conducted per aliquot of antibody obtained.
We describe a protocol for adapting the ease of the yeast surface display platform for extracellular enzymatic activity assays. The method is demonstrated using phosphorylated CD28 displayed on the yeast surface to be dephosphorylated by recombinant SHP-2 during incubation but is generalizable for many types of enzymatic modification through the modification of working buffer and enzyme-substrate pair used.
Disclosures
The authors have no conflicts of interest related to this work to disclose.
Acknowledgements
This work was supported by an NSF CAREER award to L.A.S. (CBET - 2339172) and startup funds from the University of South Florida.
In Figure 2A, microtube-open-translucent icon by Servier https://smart.servier.com/ is licensed under CC-BY 3.0 Unported https://creativecommons.org/licenses/by/3.0/. Modifications include the addition of buffer and a yeast cell (left) and the addition of antibody (center-right).
The test tube, incubator, and flow cytometer in Figure 2A were provided through www.bioicons.com under open access.
Materials
| Name | Company | Catalog Number | Comments |
| 1 L Media Bottles | Corning | 06-414-1D | |
| 1.7/2.0 mL Microtubes | Axygen | MCT-175-C | |
| 10 µL SureOne Pipet Tips | Fisher Scientific | 02-707-438 | |
| 1000 µL SureOne Pipet Tips | Fisher Scientific | 02-707-408 | |
| 12 mL Polystyrene Round-Bottom Tubes | Greiner | 07-000-212 | |
| 3 mL platic Cuvettes | BRAND | 759076D | |
| 300 µL SureOne Pipet Tips | Fisher Scientific | 02-707-411 | |
| 5 mL Serological Pipettes | Fisher Scientific | 13-678-11D | |
| Acid Casein (Casamino Acids) | Fisher Scientific | BP-1424-500 | |
| Analytical Balance | Mettler Toledo | 30243397 | |
| Bacteriological Petri Dish | Corning | Falcon 351008 | |
| Biosafety Cabinets | Labconco | Logic Class II, Type A2 302310102 | |
| Biospectrometer | Eppendorf | Kinetic 6136000010 | |
| Bovine Serum Albumin | Fisher bioreagents | BP1600-100 | |
| Citric Acid | Fisher Scientific | A940-500 | |
| CytoFLEX Flow Cytometry Analyzer | Beckam Coulter | Cytoflex C09745 | CytExpert software |
| Dextrose | Fisher Scientific | D16-1 | |
| Dithiothreitol | Fisher bioreagents | BP172-5 | |
| Donkey anti-goat FITC | Invitrogen | A16000 | |
| EDTA | Alfa Aesar | H56165.30 | |
| Ez-Link PEG4-NHS-Biotin | Thermo Scientific | A39259 | |
| Frozen-EZ Yeast Transformation II Kit | Zymo Research | T2001 | |
| Galactose | Fisher Scientific | BP656-500 | |
| General Purpose Refrigerator | Marvel Scientific | MS24RAS4RW | |
| Goat anti-myc tag antibody | Bethyl | A190-104A | |
| Mictrotube Centrifuge | Eppendorf | 5425 R 5406000313 | |
| Mini Low Temperature Refrigerated Incubator | Fisher Scientific | 15-015-2632 | |
| Mouse anti-phosphotyrosine antibody 4G10 | BioXcell | BE0194 | |
| Parafilm M | Bemis | M PM999 | |
| Phosphate Buffered Saline | Fisher bioreagents | BP399-500 | |
| Pipette Controller | Eppendorf | easypet 3 4430000018 | |
| Raffinose | Thermo Scientific | J21060-36 | |
| Recombinant human Active SHP-2 Protein | R&D Systems | 1894-SH | |
| Refrigerated Centrifuge | Eppendorf | 5910 R | |
| Saccharomyces cerevisiae yeast surface display strain EBY 100 | ATCC | MYA-4941 | |
| Shaker Incubator | Eppendorf | M1335-0002 New Brunswick Innova 42 | |
| Single Channel Pipette Set | Eppendorf | 05-403-151 | |
| Sodium Chloride | Fisher Scientific | S671-500 | |
| Sodium Citrate Dihydrate | Fisher Scientific | S279-500 | |
| Sodium Phosphate Dibasic Heptahydrate | Fisher Scientific | S373-500 | |
| Sodium Phosphate Monobasic Monohydrate | Fisher Scientific | S468-500 | |
| Streptavidin Alexa Fluor 647 | Invitrogen | S32357 | |
| Top Loading Balance | Mettler Toledo | ||
| Tris hydrochloride | EMD Millipore | 648317-100GM | |
| Tube revolver rotator | Fisher Scientific | 11-676-341 | |
| Weighing Paper | Fisher Scientific | 09-898-12B | |
| Yeast Nitrogen Base | BD Difco | 291940 | |
| Zeba Spin Desalting Columns | Thermo Scientific | 89883 |
References
- Lea, M. A., Weber, G. Role of enzymes in homeostasis: VIII. Inhibition of the activity of glycolytic enzymes by free fatty acids. J Biol Chem. 243 (6), 1096-1102 (1968).
- Mahé, M., Rios-Fuller, T. J., Karolin, A., Schneider, R. J. Genetics of enzymatic dysfunctions in metabolic disorders and cancer. Front Oncol. 13, 1230934(2023).
- Fernandez-de-Cossio-Diaz, J., Vazquez, A. A physical model of cell metabolism. Sci Rep. 8 (1), 8349(2018).
- Metallo, C. M., Vander Heiden, M. G. Understanding metabolic regulation and its influence on cell physiology. Mol Cell. 49 (3), 388-398 (2013).
- Mildvan, A. S. Mechanisms of signaling and related enzymes. Proteins. 29 (4), 401-416 (1997).
- Frosina, G. Overexpression of enzymes that repair endogenous damage to DNA. Eur J Biochem. 267 (8), 2135-2149 (2000).
- Schärer, O. D. Chemistry and biology of DNA repair. Angew Chem Int Ed. 42 (26), 2946-2974 (2003).
- de la Fuente, M., et al. Enzyme therapy: Current challenges and future perspectives. Int J Mol Sci. 22 (17), 9181(2021).
- Robertson, J. G. Enzymes as a special class of therapeutic target: clinical drugs and modes of action. Curr Opin Struct Biol. 17 (6), 674-679 (2007).
- Goddard, J. -P., Reymond, J. -L. Enzyme assays for high-throughput screening. Curr Opin Biotechnol. 15 (4), 314-322 (2004).
- Helm, J. S., Hu, Y., Chen, L., Gross, B., Walker, S. Identification of active-site inhibitors of MurG using a generalizable, high-throughput glycosyltransferase screen. J Am Chem Soc. 125 (37), 11168-11169 (2003).
- Veldhuyzen, W. F., Nguyen, Q., McMaster, G., Lawrence, D. S. A light-activated probe of intracellular protein kinase activity. J Am Chem Soc. 125 (44), 13358-13359 (2003).
- Torres, M., Forman, H. J. Encyclopedia of Respiratory. Laurent, G. J., Shapiro, S. D. , Academic Press. 10-18 (2006).
- Blume-Jensen, P., Hunter, T. Oncogenic kinase signalling. Nature. 411 (6835), 355-365 (2001).
- Martin, G. S. Cell signaling and cancer. Cancer Cell. 4 (3), 167-174 (2003).
- Lothrop, A. P., Torres, M. P., Fuchs, S. M. Deciphering post-translational modification codes. FEBS Lett. 587 (8), 1247-1257 (2013).
- Graves, J. D., Krebs, E. G. Protein phosphorylation and signal transduction. Pharmacol Ther. 82 (2), 111-121 (1999).
- Hafen, E. Kinases and phosphatases--A marriage is consummated. Science. 280 (5367), 1212-1213 (1998).
- Westphal, R. S., Anderson, K. A., Means, A. R., Wadzinski, B. E. A signaling complex of Ca2+-calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science. 280 (5367), 1258-1261 (1998).
- Barford, D., Das, A. K., Egloff, M. -P. The structure and mechanism of protein phosphatases: Insights into catalysis and regulation. Annu Rev Biophys Biomol Struct. 27, 133-164 (1998).
- Liu, Q., Qu, J., Zhao, M., Xu, Q., Sun, Y. Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacol Res. 152, 104595(2020).
- Pan, J., Zhou, L., Zhang, C., Xu, Q., Sun, Y. Targeting protein phosphatases for the treatment of inflammation-related diseases: From signaling to therapy. Signal Transduct Targeted Ther. 7 (1), 177(2022).
- Denard, C. A., et al. YESS 2.0, a tunable platform for enzyme evolution, yields highly active TEV protease variants. ACS Synth Biol. 10 (1), 63-71 (2021).
- Lim, S., Glasgow, J. E., Filsinger Interrante, M., Storm, E. M., Cochran, J. R. Dual display of proteins on the yeast cell surface simplifies quantification of binding interactions and enzymatic bioconjugation reactions. Biotechnol J. 12 (5), (2017).
- Yi, L., et al. Engineering of TEV protease variants by yeast ER sequestration screening (YESS) of combinatorial libraries. Proc Natl Acad Sci USA. 110 (18), 7229-7234 (2013).
- Yi, L., et al. Yeast endoplasmic reticulum sequestration screening for the engineering of proteases from libraries expressed in yeast. Methods Mol Biol. 1319, 81-93 (2015).
- Semenza, J. C., Hardwick, K. G., Dean, N., Pelham, H. R. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 61 (7), 1349-1357 (1990).
- Mei, M., et al. Characterization of aromatic residue-controlled protein retention in the endoplasmic reticulum of Saccharomyces cerevisiae. J Biol Chem. 292 (50), 20707-20719 (2017).
- Ezagui, J., Russell, B., Mairena, Y., Stern, L. A. Endoplasmic reticulum sequestration empowers phosphorylation profiling on the yeast surface. AIChE J. 68 (12), e17931(2022).
- Kawai, S., Murata, K. Genetic Transformation Systems in Fungi. van den Berg, M. A., Maruthachalam, K. 1, Springer International Publishing. 187-192 (2015).
- Kawai, S., Hashimoto, W., Murata, K. Transformation of Saccharomyces cerevisiae and other fungi: methods and possible underlying mechanism. Bioeng Bugs. 1 (6), 395-403 (2010).
- Loock, M., et al. High-efficiency transformation and expression of genomic libraries in yeast. Methods Protoc. 6 (5), 89(2023).
- Huang, D., Gore, P. R., Shusta, E. V. Increasing yeast secretion of heterologous proteins by regulating expression rates and post-secretory loss. Biotechnol Bioeng. 101 (6), 1264-1275 (2008).
- Yu, B., et al. Targeting protein tyrosine phosphatase SHP2 for the treatment of PTPN11-associated malignancies. Mol Cancer Ther. 12 (9), 1738-1748 (2013).
- Stern, L. A., et al. Geometry and expression enhance enrichment of functional yeast-displayed ligands via cell panning. Biotechnol Bioeng. 113 (11), 2328-2341 (2016).
- Pan, X., et al. Optimized single-cell gates for yeast display screening. Protein Eng Design Sel. 38, gzae018(2025).
- Margittai, É, et al. Production of H2O2 in the endoplasmic reticulum promotes in vivo disulfide bond formation. Antioxid Redox Signal. 16 (10), 1088-1099 (2012).
- Werner-Washburne, M., Braun, E., Johnston, G. C., Singer, R. A. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 57 (2), 383-401 (1993).
- Ezagui, J., Stern, L. A. Tyrosine phosphorylation screening on the yeast surface by magnetic bead selection and FACS. Methods Mol Biol. 2681, 275-290 (2023).
- Yarnall, M. T. N., Kim, S. H., Korntner, S., Bishop, A. C. Destabilization of the SHP2 and SHP1 protein tyrosine phosphatase domains by a non-conserved “backdoor” cysteine. Biochem Biophys Rep. 32, 101370(2022).
- Dustin, C. M., Heppner, D. E., Lin, M. J., van der Vliet, A. Redox regulation of tyrosine kinase signalling: more than meets the eye. J Biochem. 167 (2), 151-163 (2020).
- Stern, L. A., Csizmar, C. M., Woldring, D. R., Wagner, C. R., Hackel, B. J. Titratable avidity reduction enhances affinity discrimination in mammalian cellular selections of yeast-displayed ligands. ACS Comb Sci. 19 (5), 315-323 (2017).
- Waldman, A. C., Rao, B. M., Keung, A. J. Mapping the residue specificities of epigenome enzymes by yeast surface display. Cell Chem Biol. 28 (12), 1772-1779.e4 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved