Method Article
In Vivo Visualization of Calcium Transients during Fertilization and Early Development in C. elegans
In This Article
Summary
Here, we present protocols and tools for visualizing calcium during fertilization and early embryogenesis using a genetically encoded calcium reporter expressed in the germline of the model nematode C. elegans.
Abstract
Calcium is an important signaling molecule during the oocyte-to-embryo transition (OET) and early embryogenesis. The hermaphroditic nematode Caenorhabditis elegans provides several unique advantages for the study of the OET as it is transparent and has an ordered gonad that produces one mature oocyte every ~23 min at 20 °C. We have modified the genetically encoded calcium indicator jGCaMP7s to fluorescently indicate the moment of fertilization within a living organism. We have termed this reporter "CaFE" for Calcium during Fertilization in C. elegans. The CaFE reporter was engineered into a safe harbor locus in single copy, has no significant impact on physiology or fecundity, and produces a robust signal upon fertilization. Here, a series of protocols is presented for utilizing the CaFE reporter as an in vivo tool for dissecting the OET and embryogenesis. We include methods to synchronize worms, examine the effects of RNAi knockdown, mount worms for imaging, and to visualize calcium in oocytes and embryos. Additionally, we present the generation of additional worm strains to aid in this type of analysis. Demonstrating the utility of the CaFE reporter to visualize the timing of fertilization, we report that double ovulation occurs when ipp-5 is targeted by RNAi and that only the first oocyte undergoes immediate fertilization. Furthermore, the discovery of single-cell calcium transients during early embryogenesis is reported here, demonstrating that the CaFE reporter persists into early development. Importantly, the CaFE reporter in worms is simple enough to use for incorporation into course-based undergraduate research (CURE) laboratory classes. The CaFE reporter, coupled with the ordered gonad and ease of RNAi in worms, facilitates inquiry into the cell-cell dynamics required to regulate internal fertilization and early embryogenesis.
Introduction
Fertilization marks the beginning of a new life cycle, but defining the precise moment of fertilization is challenging. A conserved feature of fertilization is a wave of calcium across the oocyte immediately after sperm fusion1. Although the nature of the calcium wave, in terms of frequency and speed, differs across species, nearly all organisms exhibit a transitory increase in intracellular calcium after fertilization. The calcium wave plays a critical role in a block to polyspermy, egg activation, and other important cellular events2. Since the calcium wave initiates at the site of sperm fusion, calcium serves as a marker for fertilization3.
Caenorhabditis elegans is an ideal model organism for studying early development. The worms are transparent hermaphrodites that are genetically tractable4. Most importantly for the study of fertilization, C. elegans adults continuously produce oocytes using a strictly ordered gonad5. In fact, oocytes are the only new cells produced as the soma is post-mitotic in adulthood6. Figure 1 highlights the gonad, which consists of 2 symmetrical U-shaped tubes of developing oocytes. The oocyte closest to the spermatheca (storage organ for sperm) is termed the -1 oocyte. This assembly-line design of the worm gonad ovulates a mature oocyte into the spermatheca every ~23 min at 20 °C in young adults7. The newly fertilized embryo then moves into a shared uterus before being laid through a single vulva.
Previous techniques to visualize calcium during fertilization in C. elegans relied upon microinjection of calcium-sensitive dyes3,8. To more easily view the calcium wave upon sperm-egg fusion, a genetically encoded calcium indicator based on jGCaMP7s was inserted in single-copy at a safe harbor locus using clustered regularly interspaced short palindromic repeats (CRISPR)7,9. The reporter was termed CaFE for Calcium during Fertilization in C. elegans. The reporter shows no significant defects in physiology or fecundity.
Here, protocols are presented for visualization of the calcium wave in the oocytes and embryos of C. elegans using the CaFE reporter. Combined with the myriad tools available in the worm community, such as RNA interference (RNAi) and mCherry gonad markers, the CaFE reporter facilitates investigation into the regulation of internal fertilization events, particularly fertilization competence and the timing of fertilization. Additionally, the CaFE reporter persists into early development and is a unique tool to probe embryogenesis.
Protocol
1. C. elegans maintenance
- Nematode Growth Medium (NGM) plate preparation
- Combine 3 g of sodium chloride (NaCl), 2.5 g of Bacto-Peptone, 20 g of Agar, and 1 L of deionized water in a 4 L flask (see Table of Materials). Cover the top of the flask with aluminum foil before autoclaving.
- Autoclave to sterilize using liquid sterilization cycle settings. A sterilization cycle set at 121 °C for 30 min is recommended.
- Allow the mixture to cool to ~50 °C, approximately 20 min at room temperature (RT).
- Add 25 mL of sterile 1 M KH2PO4 (dissolved in water, pH = 6), 1 mL of sterile 1 M CaCl2 (dissolved in water), 1 mL of sterile 1 M MgSO4 (dissolved in water), and 1 mL of 5 mg/mL cholesterol (dissolved in 100% ethanol), gently swirling after each addition (see Table of Materials).
- Using a sterile technique, transfer a portion of the mixture into a sterile 300 mL beaker for easier pouring. Pour the warm media to cover the bottom of the plate.
NOTE: Approximately 10 mL per plate in 60 mm x 15 mm sterile Petri plates (see Table of Materials) will produce 100 plates. Alternatively, 35 mm x 10 mm sterile Petri dishes may be used, where ~4 mL per plate will produce ~250 plates. - Allow the NGM plates to dry at RT for at least 1 day before seeding with bacteria. Store plates upside down at 4 °C for future use.
- OP50 bacterial lawn preparation
- Make Luria Broth (LB) by combining 5 g of yeast extract, 10 g of Bacto-Tryptone, 5 g of NaCl, and 1 L of deionized water in a 2 L flask (see Table of Materials). Using a stir plate and stir bar, mix until dissolved.
- Aliquot LB into bottles and autoclave using liquid sterilization cycle settings. A sterilization cycle set at 121 °C for 30 min is recommended. If using a container with a screw cap, loosen the screw cap a quarter of a turn for proper ventilation during the autoclave cycle.
- Allow the broth to cool to RT before using.
- Using sterile technique, inoculate 50 mL of LB in a 250 mL Erlenmeyer flask with OP50 bacteria and grow overnight at 37 °C with shaking.
- Using sterile technique, transfer 300 μL of saturated culture onto the center of a 60 mm x 15 mm NGM plate. For 35 mm x 10 mm plates, 100 μL of saturated culture is recommended.
- Allow the plates to dry at RT for 1-3 days until the OP50 bacterial spot is no longer liquid. Ensure the plates are dry and are free of contamination before use. Store the plates at 4 °C for future use. Storing plates upside down reduces condensation on the plate surface.
- Worm pick construction
- Loosen the silver collar towards the head of the worm pick handle (see Table of Materials) by gently unscrewing so the four sections come apart.
- Cut 1.5 inches (about the length of the thumb) of platinum wire (see Table of Materials) using scissors and place it in the middle of the four sections; about an inch will stick out of the handle.
- Hold the wire in the center while tightening the collar; the four sections will come together in the middle and hold the platinum wire in place.
- Carefully shape the wire using a pair of flat-nose pliers. A rainbow or arc shape tends to work best for picking.
- Create about a 2-3 mm "foot" on the end of the wire by repeatedly clamping down with the flat-nose pliers. Use this "foot" to pick up the worms and keep the "foot" flat to the surface of the plate.
NOTE: Clamping too hard will cut the platinum wire. Avoid creating sharp edges using the flat-nose pliers (or equivalent) to prevent puncturing the worms or the plates.
- Worm handling
- Hold the worm pick similar to a pencil and sterilize the "foot" by placing the wire in the flame of a 95% ethanol lamp until it is bright orange.
- Place an OP50 seeded plate onto the stage of a dissecting scope and remove the lid. Gently touch the "foot" of the pick to an area of the bacterial lawn and use a gentle swiping motion to gather bacteria onto the bottom. The bacteria that are stuck to the pick (referred to as "goo") will act as a glue to pick up worms.
- Move a plate of worms to the dissecting scope stage and remove the lid while keeping the pick from touching other surfaces. Quickly and gently touch the "foot" of the pick onto a worm to pick it up. This technique enables multiple worms to be moved between plates simultaneously.
- Gently touch the "foot" of the pick to the unseeded portion of a new plate to transfer the worms; typically, this is the same plate that had the bacteria taken from it in section 1.4.2.
- Sterilize the pick between each plate and/or worm strain to avoid contamination and allow the pick to cool after sterilizing.
NOTE: Touching a hot pick on a worm will stress or kill the animal. The worm pick will cool after touching the bacterial lawn to pick up "goo" for worm transfer.
- Maintain worms at 20 °C on NGM plates (step 1.1) seeded with OP50 Escherichia coli bacteria (step 1.2). Passage worms 2 - 3 times per week by transferring several adults to fresh plates (60 mm x 15 mm) using a worm pick (steps 1.3-1.4) to avoid starvation, which can alter physiology.
NOTE: C. elegans strains and OP50 bacteria are available through the Caenorhabditis Genetics Center (CGC).
2. C. elegans developmental synchronization
- Age-synchronize worms using a timed egg-lay (described here). If a large number of worms are needed for a classroom or other purpose, perform bleach synchronization as described in Golden et al.10.
- Transfer adult worms to a new seeded OP50 plate using a platinum wire pick (described in section 1). The number of worms is dictated by the experimental need. Day 1 worms lay approximately 3 eggs per hour. Therefore, 10 worms will generate approximately 30 eggs in 1 h. Preferably, use worms on the first day of adulthood (Day 1).
- After approximately 1 h, remove the adult worms from the plate, leaving only the eggs.
NOTE: Inadvertently leaving a worm behind is a common error. Worms that remain will continue to lay eggs and disrupt synchronization. Worms mature into Day 1 adults approximately 72 h from being laid when incubated at 20°C.
3. Mounting worms for imaging
- When imaging fertilization events, use Day 1 adults to maximize visualization opportunities.
NOTE: In Day 1 adults, an oocyte is fertilized every ~23 min. However, ovulation slows with age, and a typical hermaphrodite will stop laying eggs after Day 4. - Prepare a solution of 3% agarose in water by mixing 3 g of agarose in 100 mL of water. Heat the agarose solution using a microwave until the agarose is fully dissolved. To reuse a solidified solution, heat until fully dissolved. After several uses, generate a fresh solution.
- Place a blank microscope slide for imaging in between two other microscope slides that have a single layer of lab tape across the long axis of the slide (see Table of Materials). The tape creates a spacer for the agarose pad, allowing for even distribution and a uniform width. Ensure that the three slides are in a row with the long sides touching: two slides with tape that flank a blank microscope slide.
- Drop the heated 3% agarose solution (~100-150 μL) onto the center of the microscope slide that is between the taped slides using a 1 mL pipette.
- Quickly place a new microscope slide onto the agarose droplet, ensuring the new slide is perpendicular to the slide with the agarose droplet and resting across the adjacent taped slides.
- Gently separate the slides. Avoid tearing the pad.
- Prepare the agarose pads fresh each day of imaging. It is recommended to make multiple pads when preparing for imaging, but refrain from exposing more than one pad at the same time. Exposed pads will dry out rapidly.
- Using a 20 μL pipette, add a small drop (~7 μL) of 1 mM levamisole in M9 buffer to the center of the agarose pad to paralyze the worms. Use a filter-sterilized 100 mM stock solution of levamisole to make the 1 mM working solution.
- To make M9 buffer, combine 6 g of Na2HPO4, 3 g of KH2PO4, 0.5 g of NaCl, 1 g of NH4Cl, and 1 L of deionized water in a suitable container (see Table of Materials).
- Autoclave the solution using liquid sterilization cycle settings and let the solution cool to RT before use. If using a container with a screw cap, loosen the screw cap about a quarter of a turn for proper ventilation. A sterilization cycle set at 121 °C for 30 min is recommended.
- Pick at least 5 synchronized worms and gently touch the "foot" to the levamisole droplet to transfer all worms. Use at least 5 worms per slide, as the orientation of the body can cause the intestine to obscure the germline, blocking visualization of the calcium wave in the oocyte.
NOTE: Ensure that the worms are transferred quickly as the levamisole will evaporate if left sitting on the agarose pad for longer than 5 min. - Carefully cover the agarose pad with a coverslip. Check the location of the worms, as the coverslip may cause the dispersal of the worms from the initial placement. Label the microscope slide with appropriate details, such as the name of the worm strain (see Table of Materials).
NOTE: Ensure imaging is done as soon as possible after mounting worms, as prolonged exposure to levamisole is toxic.
4. Imaging calcium during fertilization in C. elegans
- Locate the worms using a low-powered objective, such as 4x, on the microscope of choice. Use brightfield illumination.
- Use a minimum of a 20x air objective for clearly visualizing the calcium wave in oocytes. Use an immersion objective of 40x or greater.
- Depending on the system one uses, the excitation intensity typically is a fixed value. For optimal visualization of the fluorescent reporters, adjust the gain or exposure time.
NOTE: Continuous high-intensity light will damage the sample and is deleterious to worm health. If desired, turn off the laser while waiting for a fertilization event to avoid continuous GFP illumination. See the hallmarks of a fertilization event in sections 4.8 - 4.9. - Ensure the 488 nm GFP channel is set to a laser power where the CaFE reporter signal is easily visible.
- For a Nikon ECLIPSE Ti2 laser scanning confocal, detect the CaFE reporter at a laser power of 30% for the GFP channel and a gain of 75. The pinhole size for the Nikon ECLIPSE Ti2 laser scanning confocal is 30 μm.
- For the Andor Dragonfly spinning disk confocal using the Zyla camera, detect the CaFE reporter at a laser power of 30% for the GFP channel. The pinhole size for the Andor Dragonfly spinning disk confocal is 40 μm.
- Adjust the laser settings and exposure time for every sample examined.
- If using the EAG28 (CaFE; PH-mCherry) or EAG25 (CaFE; H2B-mCherry) strain, ensure the red channel is set to a laser power where the mCherry signal is easily visible in addition to the GFP channel.
- For the Nikon ECLIPSE Ti2 laser scanning confocal, detect the mCherry reporter at a laser power of 15 for the RFP channel and a gain of 130.
- For the Andor Dragonfly spinning disk confocal using the Zyla camera, detect the mCherry reporter at a laser power of 30% for the RFP channel.
- Adjust the laser settings and exposure time for every sample examined.
- Set the frame rate or frames per second (fps) as fast as the system allows.
NOTE: The CaFE reporter is typically observed at approximately 1 fps for the Nikon ECLIPSE Ti2 laser scanning confocal and 10 fps for the Andor Dragonfly spinning disk confocal with the Zyla camera. - Select time lapse or time series protocol and adjust to desired settings. Acquire time-lapse images for 30 min or less, with the repeats option set to '1'.
NOTE: If the fertilization event occurs before the time interval is complete, then stop the video to preserve file size. - Locate the worms on the slide and select a worm where the proximal gonad is visible. The intestine and the gonad twist around each other inside the worm. As such, a gonad arm is often obscured by the intestine, preventing visualization of fertilization. If the -1 oocyte is obscured, select another worm for visualization. To increase the probability of the selected worm having a visible gonad arm, mount a minimum of 5 worms per slide. Use early Day 1 adults to visualize fertilization.
- Examine each -1 oocyte in the gonad arms of the worm to determine if a fertilization event is imminent.
NOTE: Prior to fertilization, during meiotic maturation, the nucleus in the -1 oocyte will migrate to the back of the oocyte, and the nuclear envelope will dissolve. These events are visible in both Nomarksi and GFP channels using the CaFE reporter, and such events indicate that ovulation will occur within the next ~10 min. - After nuclear envelope breakdown, the cytoskeleton of the most proximal, -1 oocyte will rearrange, take on a rounded appearance, and separate from the -2 oocyte. At this point, ovulation will occur imminently. Start recording the video immediately if these events are observed. Take videos in a widefield setting.
NOTE: If movement of the nucleus or rounding of the oocyte is not visible after 20 min, an ovulation event is unlikely to occur. Focus on a different worm and/or prepare a fresh slide.
5. Quantitation of calcium transients in C. elegans fertilization
- Quantitate calcium transients from fertilization videos using the microscope system software like Nikon NIS Elements (described here). ImageJ/FIJI are also suitable for image analysis11.
- To quantitate the change in fluorescence of fertilization videos, use the following signal values: the signal at time 0, the signal from the frame of interest, and the baseline signal.
- Identify the time and frame that captures the first burst of fluorescence occurs in the -1 oocyte. Define this frame as time 0.
- To obtain the signal from the frame of interest, draw the region of interest (ROI) to encompass the following areas: the -1 oocyte prior to fertilization, the spermatheca, and the newly fertilized oocyte in the uterus. The signal displayed in the ROI is termed F1.
NOTE: Ensure the ROI covers the entire area trafficked by the oocyte. The ROI should be identical in every frame of the time-lapse recording being analyzed. - Define a blank area of the image with no fluorescence using the same dimensions as the ROI defined in step 5.4 in order to calculate the background signal in each frame. Subtract the background signal from every frame.
- To obtain the baseline signal (F0), locate the frame in a minimum of 15 frames before time 0. Using the same ROI as in step 5.4, note the signal at each frame until time 0. Average the signals (with background subtraction) from the starting frame until time 0 to calculate the baseline signal.
- Calculate the change in fluorescence (ΔF) using the following formula:
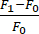 . F1 is defined in each frame as the signal in the ROI without the background signal. he F0 is the averaged baseline fluorescent signal of ≥15 frames before time 0, as calculated in section 5.6.
. F1 is defined in each frame as the signal in the ROI without the background signal. he F0 is the averaged baseline fluorescent signal of ≥15 frames before time 0, as calculated in section 5.6. - Determine the rate of change between frames by subtracting the change in fluorescence between 2 frames and dividing by the time.
6. RNAi in C. elegans
- Prepare RNAi plates
- Prepare plates according to step 1.1 and add the following additional reagents to allow for gene knockdown via RNAi at step 6.1.4.
- Add 1 mL of 1 M sterile-filtered isopropyl β-d-1-thiogalactopyranoside (IPTG) (dissolved in deionized water) for a final concentration of 1 mM IPTG in the media (see Table of Materials).
- Add 1 mL of 100 mg/mL sterile-filtered ampicillin (dissolved in deionized water) for a final concentration of 100 μg/mL ampicillin in the media (see Table of Materials). Alternatively, use 40 μg/mL carbenicillin12.
- Swirl gently but thoroughly to mix and pour plates as described in steps 1.1.4 - 1.1.5.
- Preparation of RNAi-saturated culture
- Calculate the amount of saturated culture by multiplying the number of seeded RNAi plates needed by 300 μL when using 60 mm x 15 mm plates or by 100 μL when using 35 mm x 10 mm plates.
- Using a sterile technique, grow desired RNAi bacteria in LB (described in steps 1.2.1-1.2.3) containing 100 μg/mL ampicillin overnight at 37 °C until saturated. Include a bacterial strain containing the L4440 empty vector as a negative control and a bacterial strain containing an RNAi positive control (e.g., egg-5) to verify the RNAi is working. Additionally, include a "blank" condition containing only LB to verify there is no contamination in the stock LB.
- Add sterile-filtered IPTG to a final concentration of 1 mM to the saturated overnight culture for induction of dsRNA expression and incubate for at least 2 h 30 min at 37 °C before seeding. Alternatively, dilute back the saturated overnight culture out of the log-phase prior to inducing with IPTG.
NOTE: IPTG is included in both the plates and the saturated culture for optimal dsRNA expression. - Using sterile technique, transfer 300 μL of the induced culture onto the center of a 60 mm x 15 mm RNAi plate (solidified). Alternatively, use 100 μL of saturated culture for a 35 mm x 10 mm plate.
- Allow the plates to dry at RT for 1-3 days until the RNAi bacterial spot is no longer liquid. Ensure plates are dry and are free of contamination before use. Store plates at 4 °C upside down for future use.
NOTE: Use RNAi plates as soon as possible for optimal knockdown, as RNAi efficacy decreases over time. Discard if >1 month old.
- As described in section 2, synchronize the worms onto RNAi plates.
- Check worms 1-2 days after the egg-lay synchronization for developmental delays.
- Some RNAi may cause developmental defects when the knockdown begins at egg-lay. If needed, place worms onto the RNAi plates at a later stage, such as L3.
- As described in sections 3 and 4, mount the worms and prepare for imaging using any microscope system equipped to detect GFP fluorescence.
7. Imaging calcium during early embryogenesis in C. elegans
- Synchronize worms as described in section 2. Use the EAG28 strain, which combines the CaFE reporter and a pleckstrin-homology domain-mCherry marker highlighting the plasma membrane boundary. Alternatively, to visualize fertilized embryos ex utero, gently cut open the worm along the uterus using a sterile syringe needle to release the embryos.
- Prepare worms or embryos for imaging as described in section 3, and follow the same instructions described in section 4 for setting imaging parameters.
- Center the area of interest on the uterus of the worm to visualize embryos or a laid egg.
- For embryos in utero, ensure that the uterus is not behind the intestine, the uterus is not crowded, and that the individual cells in the embryo are clear in the focal plane.
- For eggs ex utero, ensure that the stages of the cells are not past the comma stage of the worm life cycle.
- Select time lapse or time series protocol and adjust to desired settings.
- For optimal visualization of embryo calcium transients, increase the time settings for prolonged movies. Keep in mind that constant exposure to the laser will harm the embryo over long periods of time.
- Capture videos or images by focusing on cells in embryos in the desired cell stage.
- Use a 40x immersion objective or greater for imaging, although the transients are readily detected using a 20x air objective.
Results
Using the protocols outlined in this manuscript, the dynamic patterns of calcium signaling in fertilization and embryogenesis were observed in C. elegans.
A typical fertilization sequence in worms containing the CaFE reporter is shown in Figure 2. To facilitate analysis, the EAG28 strain was used which combines the CaFE reporter with a pleckstrin homology domain (PH-mCherry) transgene13. The PH-mCherry marker localizes to the plasma membrane and allows for easier visualization of cell boundaries, particularly in the gonad arm. The EAG28 strain was created by crossing the EAG16 strain containing the CaFE reporter with the OD70 PH-mCherry reporter strain13. In C. elegans, ovulation and fertilization occur concurrently. Time 0 of fertilization is considered the first frame where a fluorescence signal is detected in the ovulating oocyte (Figure 2). A bright burst of fluorescence occurs at the site of sperm fusion as soon as the leading edge of the oocyte enters the spermatheca, the storage organ for sperm7. The signal appears before ovulation is complete. The calcium wave is biphasic, with a rapid initial burst followed by a wave of fluorescence from the entry point toward the opposite pole. The entire oocyte becomes fluorescent in <30 s.
Note that the CaFE reporter is in a single copy, and therefore, the signal is not as bright as other transgenes. Z-stack time-lapse is not recommended during ovulation unless a spinning disk confocal is available and optimized. Z stack images can be obtained before or after a movie to capture 3D changes in gonad morphology. Although the images here were taken with a confocal microscope, the transients have also been observed using widefield fluorescence microscopy which is more common and affordable than confocal. Using a laser scanning confocal, the frame rate is approximately 1 fps. Using a spinning disk confocal, the frame rate is 10 fps or faster. The background signal of the CaFE reporter is sufficient to illuminate the maturing oocytes in the gonad arm. Upon fertilization, a 1.5-2x increase in GFP signal intensity is typically observed.
Several alternative methods exist for quantitation. For example, the Imaris image analysis software, created by Oxford Instruments (the same parent company as the Andor Dragonfly spinning disk confocal microscope), has the ability to quantitate the signal in each frame from only the transiting oocyte instead of a bigger ROI. However, the Imaris software is not free. Additionally, a detailed image analysis strategy to measure calcium waves in the oocyte has been described in excellent detail by Takayama, Fujita, and Onami and uses ImageJ, which is freely available14. Alternatively, kymographs to illustrate the wave of fluorescent signal across a single oocyte require more extensive image manipulation than described here but are described in Takayama and Onami3.
A distinct advantage of C. elegans is its ability to knockdown the expression of nearly any gene by feeding the worms bacteria expressing dsRNA to trigger endogenous RNAi15,16. For optimal dsRNA expression, both the RNAi plates and the saturated RNAi culture contain IPTG. The dsRNA is produced by expressing the gene of interest with flanking promoters. Here, we have used the Ahringer library which targets most of the worm genome through the use of 16,256 bacterial strains17. Alternatively, an ORFeome of ~11,000 RNAi clones was created by the Vidal lab using the Gateway system and is available for purchase through Horizon Discovery18.
To demonstrate the utility of the CaFE reporter in combination with RNAi, we examined premature ovulation. A partial deletion in ipp-5 induces a double ovulation event where both the -1 oocyte and the -2 oocyte enter the spermatheca during the same ovulation19. IPP-5 is a phosphatase that acts on IP3 and effectively decreases levels of the IP3 second messenger. We found that RNAi knockdown of ipp-5 elicits a double ovulation phenotype similar to the mutant.
Analysis of the CaFE reporter during the double ovulation induced by ipp-5 RNAi knockdown revealed novel aspects of the OET. First, a calcium signal was observed immediately upon entry into the spermatheca in the leading -1 oocyte but not in the trailing -2 oocyte (Figure 3). Although 2 oocytes are ovulated, the -1 oocyte is still the only oocyte that shows signs of proper maturation, in particular, the breakdown of the nuclear envelope (NEBD). These data suggest that even though the -2 oocyte is in the presence of sperm, it is not competent to be fertilized as it has not yet properly matured. Second, the -2 oocyte exhibits a delayed calcium wave, typically when the oocyte exits the spermatheca for the uterus. Although oocyte maturation is normally a prerequisite for ovulation, these data suggest that the maturation of the oocyte and competence for fertilization can still occur after ovulation. The visualization of the delayed calcium wave during ipp-5 knockdown highlights the advantages of the CaFE reporter to interrogate fertilization competence and timing.
Furthermore, we have discovered that the CaFE reporter is detectable in laid eggs and reveals single-cell calcium transients during early embryogenesis in C. elegans. Embryos from synchronized Day 1 adult EAG28 (CaFE; PH-mCherry) worms were imaged using the protocols from sections 1-5 with the Andor Dragonfly spinning disk confocal using the Zyla camera (Figure 4). The calcium transients are confined to single cells and take less time than the fertilization calcium wave to complete (~9 s, n=11). However, the calcium wave during embryogenesis does not appear to be biphasic. Calcium transients were not observed before the 8-cell stage. Notably, calcium does not localize to the cleavage furrow in C. elegans, as is seen in many other organisms, including humans and Xenopus20,21,22. Single-cell calcium transients were observed well after gastrulation (~200 min after fertilization) and in laid eggs. A single embryo displays multiple calcium transients over time but in different cells and typically one cell at a time. Note that continuous exposure to laser stimulation will damage the embryos. Either lower LED/laser power or discontinuous exposure is recommended. However, since the calcium waves are relatively short at ~9 s, we do not recommend slower than 2 s between LED/laser stimulation events.
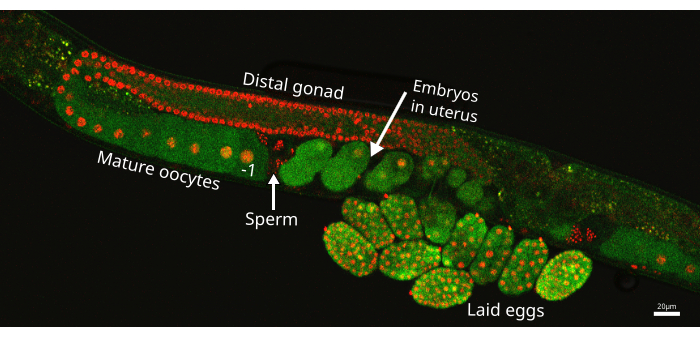
Figure 1: C. elegans gonad arm. Image of one gonad arm from the EAG25 worm strain, showing the CaFE reporter GFP (green) and the histone 2B-mCherry marker (red), which highlights nuclei in the germline. The proximal oocyte is denoted by the -1 directly before the spermatheca, where the sperm are stored (arrow). The embryo located closest to the spermatheca but inside the uterus is the most recent embryo. The image was taken using the Nikon ECLIPSE Ti2 laser scanning confocal. Scale bar = 20 µm. Please click here to view a larger version of this figure.
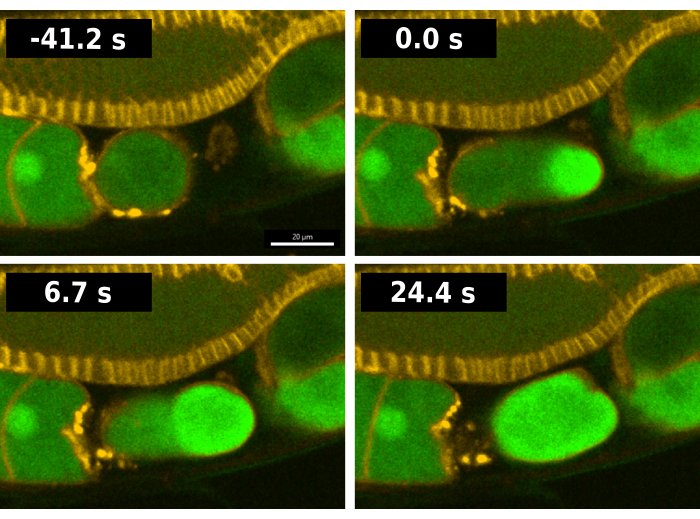
Figure 2: Visualization of the calcium wave during fertilization. Time series images of fluorescence reflecting intracellular calcium during fertilization in the EAG28 worm strain. The EAG28 strain contains both the CaFE reporter (green) and a pleckstrin-homology domain-mCherry fusion (yellow), which highlights plasma membranes. Time 0 indicates the first frame portraying a clear increase in fluorescent signal in the oocyte. Time 24.4 s reflects the first frame showing whole oocyte fluorescence. Time series images were taken using the Andor Dragonfly spinning disk confocal with a Zyla camera. Scale bar = 20 µm. Please click here to view a larger version of this figure.
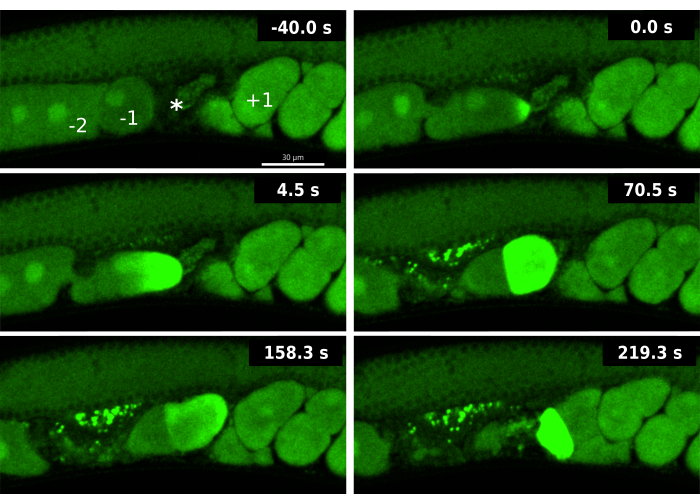
Figure 3: Calcium waves during an ipp-5 RNAi-induced double ovulation event. Time-lapse images of sequential calcium waves of the -1 oocyte and -2 oocyte in an EAG16 strain (CaFE in GFP green) exposed to ipp-5 RNAi. The -40.0 s frame includes labels for the -1 oocyte (-1), -2 oocyte (-2), spermatheca (*), and +1 embryo (+1). Time 0 s displays the initial increase in fluorescence of the calcium wave of the -1 oocyte. Time 4.5 s shows the calcium wave spreading towards the opposite pole in the -1 oocyte. Time 70.5 s displays whole cell fluorescence in the -1 oocyte accompanied by the -2 oocyte at baseline GFP signal; both oocytes are in the spermatheca. The fertilized -1 oocyte and unfertilized -2 oocyte enter the uterus at time 158.3 s. At 219.3 s, the -2 oocyte displays a late fluorescent calcium wave. Images were taken using the Andor Dragonfly spinning disk confocal with a Zyla camera. Scale bar = 30 µM Please click here to view a larger version of this figure.
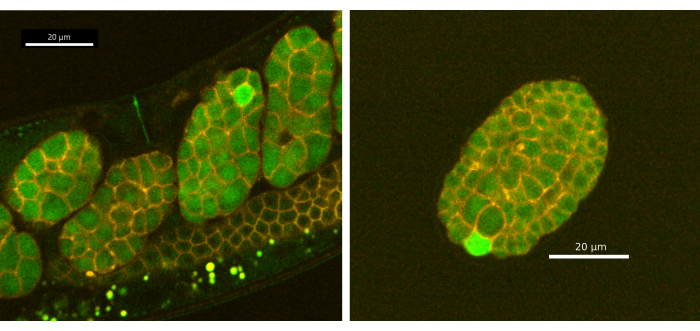
Figure 4: Single-cell calcium transients in early embryogenesis. (A) Embryos in utero and (B) ex utero (laid egg) displayed a fluorescent signal in a single cell during embryogenesis. Images were taken using the Andor Dragonfly spinning disk confocal with a Zyla camera. Scale bar = 20 µm. Please click here to view a larger version of this figure.
Discussion
A simple tool with a robust protocol is a potent combination to tackle difficult scientific questions. Here, methods for the visualization of calcium as an easily detectable proxy for fertilization are presented using the CaFE reporter. This same reporter persists into early embryogenesis and also allows the visualization of calcium transients further into development. Calcium signaling serves as a critical second messenger that demarcates major shifts in cellular function, particularly for developmental biology. In the oocyte, the first burst phase of the calcium wave marks not just the timing of fertilization but also the site of sperm fusion3. In C. elegans, the A-P axis is also determined by the site of sperm fusion23. Therefore, the ability to visualize calcium in oocytes and embryos allows for the investigation of complex questions that are central to cell and developmental biology.
The method described here with the CaFE reporter should be straightforward enough for nematode novices. Previous methods to detect calcium waves in oocytes of C. elegans relied upon dye injection3,8,14. While these studies were important and illuminating, dye injection is labor-intensive, and injection equipment is not available in every lab.
The protocols presented here have been optimized for healthy worms. To maximize the chance for success, ensure that there is no contamination on the worm media or in the bacteria lawn on which the worms feed. Do not expose the worms to stress conditions, such as temperature, as these will affect ovulation and fertilization. Additionally, controls for RNAi efficacy should be included with every experiment as efficacy decreases over time. Use a positive control for RNAi efficacy like egg-5, which generates embryos, but no viable progeny as eggshell formation is compromised24. Furthermore, the image capture parameters must be optimized for each microscopy system. Our specifications are included here as a reference, but deviations are to be expected. Although this system readily detects cytoplasmic calcium as a proxy for fertilization, it does not necessarily represent a bona fide sperm-oocyte fusion event.
The wide array of tools and mutants freely distributed within the worm community enhance the utility of the CaFE reporter. The reporter is integrated into the worm genome and is easily crossed into other mutant or reporter C. elegans strains25. Reported here is the creation of EAG25 expressing the CaFE reporter with a histone H2B-mCherry marker to visualize nuclei (Figure 1) and EAG28 with both the CaFE reporter and a pleckstrin-homology domain-mCherry marker, which highlights the cell periphery (Figure 2)13,26. Both strains aid in the visualization of cells in the germline and during embryogenesis. Furthermore, the facility of RNAi in
C. elegans, when used with the CaFE reporter, have revealed new insights into fertilization competence. As shown in the double ovulation induced by ipp-5 RNAi knockdown in Figure 3, the presence of sperm and an ovulated oocyte is insufficient to stimulate a fertilization event.
These results indicate that another signal, or the absence of an inhibitor, must exist that allows the oocyte to become fertilized. The prematurely ovulated oocyte displays a delayed calcium transient when the oocyte moves into the uterus. This late calcium wave suggests that the prematurely ovulated -2 oocyte can develop fertilization competency with time. We anticipate that studies on the timing of fertilization, particularly with regard to cell-cell signaling and regulation, will be aided by the use of the CaFE reporter. Additionally, the CaFE reporter persists into embryogenesis and displays single-cell calcium transients. This embryonic calcium signaling was also reported within the first 24 hours of zebrafish development27. The role of the calcium transients is unknown, but the presence suggests a cell signaling event during development that has not yet been explored. Notably, the calcium transients were not observed in newly fertilized zygotes. Therefore, calcium does not localize to the cleavage furrow as has been documented in several other organisms, including Xenopus and humans20,21,22.
Importantly, the CaFE reporter is easy enough to be used by undergraduates with minimal training. We have designed and executed a 1-credit CURE (course-based undergraduate research experience) lab for biology students with the strains and protocols described here. Over the course of a semester, the class met once a week for 3 h or twice a week for 1 h 30 min each. Students were given the choice to work by themselves or in groups of 2. Each student/pair selected a different gene to study from a curated list. They performed RNAi against their chosen gene in the EAG28 strain and examined worms for effects on fecundity, fertilization, and/or gonad morphology. Based on their results and their background reading using primary literature, the students developed hypotheses that they could test in subsequent experiments. This iterative design was critical to increasing student engagement28. The students obtained authentic research experience and gained skills in model organisms, genetic screens, and fluorescence microscopy. Given the ease of use of the CaFE reporter, students with no research experience were able to succeed. Afterward, the students overwhelmingly expressed a preference for the CURE format over traditional lab classes, with many students expressing a desire to continue with research. Taken together, these tools and protocols aid in both education and research into early developmental processes.
Disclosures
The authors declare no competing or financial interests.
Acknowledgements
KSKG was funded by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R15HD111986). Some strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD4010440). We thank WormBase.
Materials
| Name | Company | Catalog Number | Comments |
| Agar | Fisher Scientific | DF0140-07-4 | 2 kg; Powder dissolves easier than flakes |
| Agarose | MidSci | BE-A125 | 500 g |
| Alcohol lamp | Fisher Scientific | S13475 | Use with 95% ethanol |
| Ammonium chloride (NH4Cl) | Fisher Scientific | AAA1500030 | 250 g |
| Ampicillin | Fisher Scientific | BP1760-5 | 5 g |
| AMSCO 400 Series Small Steam Sterilizer | Steris Healthcare | N/A | |
| Bacto-peptone | Fisher Scientific | BP1420-500 | 500 g |
| Bacto-tryptone | Fisher Scientific | DF0123-17-3 | 500 g |
| Calcium chloride (CaCl2) | Fisher Scientific | C69-500 | 500 g |
| Cholesterol | Thermo Scientific | A11470.18 | 50 g |
| Dragonfly 200 spinning disk confocal | Oxford Instruments Andor | N/A | Used with Leica microscope |
| Fisherbrand Superfrost Cytogenics Microscope Slides | Fisher Scientific | 22-035-900 | 144 slides per pack |
| Flat Nose Pliers, Smooth Jaw | Home Depot | 305530604 | Ensure pliers are smooth jaw |
| Isopropyl β- d-1-thiogalactopyranoside (IPTG) | Fisher Scientific | BP1755-10 | 10 g; dioxane-free |
| Laboratory tape | Fisher Scientific | 15-901-10R | 0.5 inch tape is used to tape microscope slides |
| Levamisole | Fisher Scientific | AC187870100 | 10 g |
| Magnesium sulfate (MgSO4) | Fisher Scientific | M63-500 | 500 g |
| Microscope cover glass | Fisher Scientific | 12541016 | 1 oz pack |
| Nikon ECLIPSE Ti2 laser scanning confocal | Nikon | N/A | |
| Nikon NIS Elements software | Nikon | N/A | Confocal |
| OP50 Escherichia coli | Caenorhabditis Genetics Center (CGC) | OP50 | |
| Platinum Wire | TriTech | PT-9010 | |
| Potassium phosphate dibasic (K2HPO4) | Fisher Scientific | P288-500 | 500 g |
| Potassium phosphate monobasic (KH2PO4) | Fisher Scientific | AA1159436 | 500 g |
| Sodium chloride (NaCl) | Fisher Scientific | S271-500 | 500 g |
| Sodium phosphate dibasic heptahydrate (Na2HPO4) | Fisher Scientific | S471-3 | 3 kg |
| Stereo microscope | Leica | KL300 LED | |
| Sterile Petri dish (35 mm x 10 mm) | CellTreat | 229638 | 960 Petri dishes per case |
| Sterile Petri dish (60 mm x 15 mm) | CellTreat | 229665 | 500 Petri dishes per case |
| Strain EAG16 spn-4p::jGCaMP7s::pie-1u | Caenorhabditis Genetics Center (CGC) | EAG16 | Created by Kim Guisbert Lab |
| Strain EAG25 spn-4p::jGCaMP7s::pie-1u; ujIs113 II. | Caenorhabditis Genetics Center (CGC) | EAG25 | Created by Kim Guisbert Lab |
| Strain EAG28 spn-4p::jGCaMP7s::pie-1u; unc-119(ed3) III; ltIs44 V. | Caenorhabditis Genetics Center (CGC) | EAG28 | Created by Kim Guisbert Lab |
| Strain JIM113 ujIs113 II [pie-1p::mCherry::H2B::pie-1 3'UTR + nhr-2p::his-24::mCherry::let-858 3'UTR + unc-119(+)] | Caenorhabditis Genetics Center (CGC) | JIM113 | Created by E. Preston - Murray Lab |
| Strain OD70 unc-119(ed3) III; ltIs44 V [pie-1p::mCherry::PH(PLC1delta1) + unc-119(+)] | Caenorhabditis Genetics Center (CGC) | OD70 | Created by Audhya/Oegema - Greenstein Lab |
| Tritech Worm Pick Handle | TriTech | TWPH1 | |
| Yeast extract | IBI Scientific | IB49160 | 500 g |
References
- Stein, P., Savy, V., Williams, A. M., Williams, C. J. Modulators of calcium signalling at fertilization. Open Biol. 10 (7), 200118 (2020).
- McAvey, B. A., Wortzman, G. B., Williams, C. J., Evans, J. P. Involvement of calcium signaling and the actin cytoskeleton in the membrane block to polyspermy in mouse eggs. Biolo Reprod. 67 (4), 1342-1352 (2002).
- Takayama, J., Onami, S. The sperm TRP-3 channel mediates the onset of a Ca 2+ wave in the fertilized C. elegans oocyte. Cell Rep. 15 (3), 625-637 (2016).
- Yamamoto, I., Kosinski, M. E., Greenstein, D. Start me up: Cell signaling and the journey from oocyte to embryo in C. elegans. Dev Dyn. 235 (3), 571-585 (2006).
- Hubbard, E. J. A., Greenstein, D. The Caenorhabditis elegans gonad: A test tube for cell and developmental biology. Dev Dyn. 218 (1), 2-22 (2000).
- Korta, D. Z., Hubbard, E. J. A. Soma-germline interactions that influence germline proliferation in Caenorhabditis elegans. Dev Dyn. 239 (5), 1449-1459 (2010).
- Toperzer, K. M., Brennan, S. J., Carroll, D. J., Guisbert, E. A., Kim Guisbert, ., S, K. Visualization of the biphasic calcium wave during fertilization in Caenorhabditis elegans using a genetically encoded calcium indicator. Biol Open. 12 (9), 059832 (2023).
- Samuel, A. D., Murthy, V. N., Hengartner, M. O. Calcium dynamics during fertilization in C. elegans. BMC Dev Biol. 1 (1), 8 (2001).
- Dana, H., et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods. 16 (7), 649-657 (2019).
- Golden, N. L., Plagens, R. N., Kim Guisbert, K. S., Guisbert, E. Standardized methods for measuring induction of the heat shock response in Caenorhabditis elegans. J Vis Exp. (161), e61030 (2020).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9 (7), 676-682 (2012).
- Kamath, R., Ahringer, J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 30 (4), 313-321 (2003).
- Kachur, T. M., Audhya, A., Pilgrim, D. B. UNC-45 is required for NMY-2 contractile function in early embryonic polarity establishment and germline cellularization in C. elegans. Dev Biol. 314 (2), 287-299 (2008).
- Takayama, J., Fujita, M., Onami, S. In vivo live imaging of calcium waves and other cellular processes during fertilization in Caenorhabditis elegans. Bio Protoc. 7 (7), e2205 (2017).
- Fire, A., Xu, S., Montgomery, M. K., Kostas, S. A., Driver, S. E., Mello, C. C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 391 (6669), 806-811 (1998).
- Conte, D., MacNeil, L. T., Walhout, A. J. M., Mello, C. C. RNA Interference in Caenorhabditis elegans. Curr Protoc Mol Biol. 109, 1-30 (2015).
- Kamath, R. S., et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 421 (6920), 231-237 (2003).
- Reboul, J., et al. elegans ORFeome version 1.1: experimental verification of the genome annotation and resource for proteome-scale protein expression. Nat Genet. 34 (1), 35-41 (2003).
- Bui, Y. K., Sternberg, P. W. Caenorhabditis elegans inositol 5-phosphatase homolog negatively regulates inositol 1,4,5-triphosphate signaling in ovulation. Mol Biol Cell. 13 (5), 1641-1651 (2002).
- Muto, A., Kume, S., Inoue, T., Okano, H., Mikoshiba, K. Calcium waves along the cleavage furrows in cleavage-stage Xenopus embryos and its inhibition by heparin. J Cell Biol. 135 (1), 181-190 (1996).
- Paudel, S., Sindelar, R., Saha, M. Calcium signaling in vertebrate development and its role in disease. Int J Mol Sci. 19 (11), 3390 (2018).
- Paudel, S., Yue, M., Nalamalapu, R., Saha, M. S. Deciphering the calcium code: A review of calcium activity analysis methods employed to identify meaningful activity in early neural development. Biomolecules. 14 (1), 138 (2024).
- Goldstein, B., Hird, S. N. Specification of the anteroposterior axis in Caenorhabditis elegans. Development. 122 (5), 1467-1474 (1996).
- Johnston, W. L., Dennis, J. W. The eggshell in the C. elegans oocyte-to-embryo transition. Genesis. 50 (4), 333-349 (2012).
- Stevenson, Z. C., Moerdyk-Schauwecker, M. J., Jamison, B., Phillips, P. C. Rapid self-selecting and clone-free integration of transgenes into engineered CRISPR safe harbor locations in Caenorhabditis elegans. G3. 10 (10), 3775-3782 (2020).
- Zacharias, A. L., Walton, T., Preston, E., Murray, J. I. Quantitative differences in nuclear β-catenin and TCF pattern embryonic cells in C. elegans. PLoS Genet. 11 (10), e1005585 (2015).
- Webb, S. E., Miller, A. L. Calcium signalling during zebrafish embryonic development. BioEssays. 22 (2), 113-123 (2000).
- Wiseman, E., Carroll, D. J., Fowler, S. R., Guisbert, E. Iteration in an inquiry-based undergraduate laboratory strengthens student engagement and incorporation of scientific skills. J Scholarsh Teach Learn. 20 (2), 99-112 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved